Empatica unveils EmbraceMini, the world’s smallest actigraphy wearable for clinical trials
Boston, MA – Empatica, a pioneer in digital biomarker development and patient monitoring using wearables and AI, today unveiled its revolutionary new device for clinical trials, EmbraceMini.
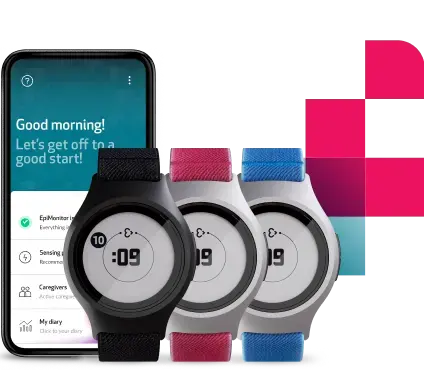
EmbraceMini is an ultra-compact health monitoring device, focused on delivering exceptional wearability and high-quality data without compromising sponsor or participant needs. With its incredibly small footprint and refined looks, EmbraceMini offers effortless, seamless and non-invasive activity data collection while prioritizing the participant experience at all times. It wirelessly transmits sensor data to the FDA-cleared Empatica Health Monitoring Platform, enabling the continuous analysis and extraction of precise digital biomarkers from clinical trial participants while boosting compliance.
In a statement, our CTO & Co-founder, Simone Tognetti, describe how EmbraceMini was designed to address a gap in DHTs, where high data integrity and value meet unprecedented participant comfort:
“We’ve created a wearable that combines the reliability of a medical device with the accessibility of a design accessory, something previously missing from the market. Most devices adopted in clinical trials today are too bulky, or just not fit for purpose. Wearability is crucial for the success of the study, so our goal with EmbraceMini was to make a compact, beautiful wearable that offers the same data quality and range as EmbracePlus, without ‘competing’ with people’s favorite watches or accessories for room on their wrist.”
At just 12mm thick and 14mm wide, EmbraceMini is the smallest wristworn actigraphy device for clinical research in the world–the size of a USB flash drive or AA battery. It is purpose-built for studies that monitor sleep and movement, featuring a long battery life that can achieve at least 7 days of continuous data collection via 4 high-frequency sensors: a 3-axis Accelerometer, a 3-axis Gyroscope, a 3-axis Magnetometer, and an Ambient Light sensor.
EmbraceMini can accurately and passively monitor over 200 digital sleep and activity measures, thanks to sophisticated algorithms developed by Empatica and its partners. Among other important metrics, EmbraceMini can track digital biomarkers across physical activity, sleep, gait, and light exposure, which can serve a key role as digital endpoints in studies monitoring sleep disorders, movement disorders, obesity, depression, pain, and more. EmbraceMini can also collect raw data, providing researchers with more transparency and flexibility in how they process information.
Thanks to Empatica’s modular approach, EmbraceMini can be worn on the wrist, leg, around the waist, or the ankle. Empatica also recently announced its double-device functionality, allowing the simultaneous collection of data from two devices worn by the same participant. With the arrival of EmbraceMini, researchers can combine data from Empatica’s flagship wearable EmbracePlus (including cardiorespiratory measures collected from its PPG sensor) and EmbraceMini, or two EmbraceMini devices, worn on different locations of the body.
EmbraceMini works seamlessly with Empatica’s software, and can also be integrated in existing Clinical Trial Management Systems using a Cloud API.
If you are interested in learning more about the device and to request a demo, visit empatica.com/embracemini or write to us at research@empatica.com.