Improving clinical trial compliance with site and patient centered technology
Adoption of digital health technologies (DHTs) in clinical trials is growing, particularly within decentralized and hybrid trial designs. According to current projections, 8% of trials set to complete in 2025 [1] include some form of virtual component, and approximately one-third of those trials use wearable technologies.
The most common use cases are in CNS and neurology studies, where the day-to-day variability of symptoms makes continuous, objective data especially valuable. In these therapeutic areas, digital measures like actigraphy can provide insights that traditional assessments often miss. Studies show that DHTs can reduce trial costs by 10-14%, shorten timelines by 4-5 months, and even improve participant retention [2]. Yet despite this, adoption still lags far behind expectations.
Back in 2018, analysts predicted that 70% of trials would include wearable sensors by 2025. Today, the actual number is closer to 7-8% (Figure 1) [3]. This gap isn’t due to a lack of interest; it’s due to practical barriers: operational complexity, inconsistent compliance, integration difficulties, and limited confidence in data from unvalidated tools.
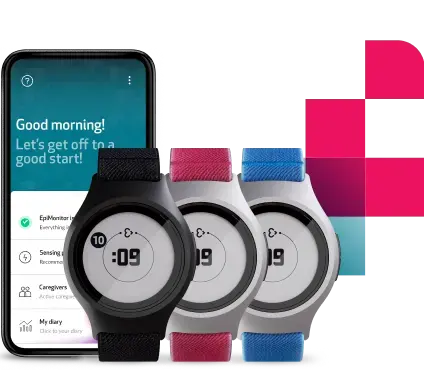
In this blog, we explore what sites and patients actually need from trial technology to use it, why reducing friction is critical to compliance and adoption, and how we approached these challenges in designing EmbraceMini, a wearable built to support modern clinical trials without disrupting them.
Complexity is the hidden cost for sites and sponsors
The feedback from site staff often centers around setup time, training, and ongoing support.
Sites are often juggling multiple platforms, protocols, and endpoints. Adding a digital health wearable can feel like just one more system to manage, especially if it's not intuitive or well integrated. Sponsors need data that is robust and regulatory-ready, but if a device requires complex onboarding or constant troubleshooting and site intervention, it becomes a burden.
And when site staff feel uncertain or overwhelmed, that tension can quickly filter down to participants.
Patients want comfort, not complications
From the patients’ side, the story is equally clear. Across dozens of deployments, the most consistent feedback has been about wearability and ease of use.
If a device feels bulky, too medical, or difficult to manage, participants are less likely to wear it consistently, especially in longer-term studies. Confusing setup, unclear instructions, or even a charging schedule that doesn’t align with daily routines can lead to drop-off.
No matter how advanced the tech is, it has to fit seamlessly into real life. Otherwise, compliance becomes a struggle.
Solving everyday challenges in actigraphy-based clinical trials with EmbraceMini
Adopting actigraphy-based measures in clinical trials brings incredibly valuable insights into sleep, physical activity, and detailed motion patterns like gait, but it also introduces real-world challenges that can impact compliance, data quality, and patient retention. EmbraceMini was designed to directly address these barriers through thoughtful design and operational simplicity without trade-offs.
Here’s how we addressed the key friction points:
Bulky, uncomfortable wearables are one of the most common reasons for poor compliance in clinical trials
EmbraceMini’s compact, lightweight design improves comfort and wearability, making it easier for participants to keep the device on throughout the day and over longer study periods. Beyond its discreet wrist-worn profile, EmbraceMini isn’t limited to a single form; it can be worn on the ankle, shoe, or hip using dedicated accessories. This flexibility allows the device to adapt to different study protocols and participant preferences, without compromising on data quality or sensor performance.
Wrist space is limited, and participants often want to keep wearing their personal devices
EmbraceMini is designed to be worn alongside a smartwatch or traditional wristwatch, and it can also be placed on alternate body locations like the ankle, shoe, or waist, giving researchers more flexibility without compromising data quality.
Complex implementation and high support needs can slow down deployment and increase costs
By simplifying onboarding, automating data flow through Empatica’s Care App, and integrating seamlessly into the Empatica Health Monitoring Platform, EmbraceMini helps reduce site workload and operational overhead. And with Empatica’s Cloud API, EmbraceMini data can flow directly to an existing Clinical Trial Management System, further reducing site burden.
A poor patient experience often leads to early drop-off, especially for longer studies
EmbraceMini was designed to minimize that risk. Its long battery life reduces the need for daily charging, and data flows automatically via the Care App, with no action required from the participant. On the rare occasion that support is needed, the app provides clear, step-by-step guidance. By staying largely invisible in day-to-day life, EmbraceMini reduces burden and helps keep participants engaged throughout the trial.
Technology can bring enormous value to clinical trials, but only when it fits into the realities of how studies actually run.
What we’ve learned through years of working with the world’s top research institutions is that the details matter. The way a device feels, how long it takes to set up, and whether it blends into daily routines or disrupts them. These are often the deciding factors between a tool that gets used and one that doesn’t.
With EmbraceMini, our goal wasn’t simply to add more features, but to remove more friction: for sites, for participants, and for the teams running trials day to day.
🎥 Want to see it in action?
Watch the EmbraceMini webinar on demand or reach out to learn more about how it fits into your study.
References:
[3] DT Consulting, Clinical Trial Digital Tracker Q3‑2022 (Apr 2023), https://dt-consulting.com/dts-clinical-trial-digital-tracker-q3-2022/ ; Marra & Stern, 2024, https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.3192