Empatica to deploy a COVID-19 early detection wearable
Empatica’s efforts to combat the Covid pandemic continue!
In June, we announced our partnership with U.S. Department of Health and Human Services (HHS) to validate the COVID-19 detection capabilities of Empatica’s algorithm, Aria, on at-risk healthcare workers.
Today, we are thrilled to announce that we have been selected by the U.S. Army Medical Research and Development Command (USAMRDC) to deploy a wearable and algorithm that enable the early and pre-symptomatic detection of COVID-19. We were chosen from a rigorous selection process, following a response to the Request for Project Proposals issued this May 2020.
The aim: protecting the health of the general population by preventing outbreaks via early detection and alerting.
The path towards deployment
The scope of the initiative is multi-phased, beginning with a clinical validation study of the Empatica Aria algorithm on healthy participants at risk of contracting SARS-CoV-2.
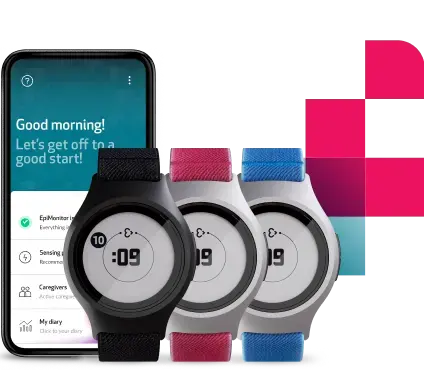
Using physiological data collected by our medical smartwatch, the EmbracePlus, the Aria algorithm gives a daily risk-indication of an individual having contracted COVID-19.
Over the course of the validation, relevant FDA regulatory approvals will run in parallel. The project will conclude with end-user testing in a decentralized clinical study to validate components and requirements of the entire platform.
The consumer online clinical study will be supported by an Investigational Device Exemption (IDE) that will be activated for this purpose. The final tested platform (EmbracePlus running on the Empatica RPM platform with the Aria algorithm) will be tested on a target population of 300 people.
The right wearable for the job
The EmbracePlus, soon to be released, is a medical device, elegantly looking like a smartwatch, and featuring clinical-grade sensors that measure pulse rate, pulse rate variability, temperature, and respiratory rate, as well as electrodermal activity.
The device will unobtrusively monitor a person’s vital signs, and check and alert for patterns that suggest the presence of a COVID-19 infection inside a dedicated app. This will be used to help wearers self-isolate and seek testing without unwittingly infecting others.
EmbracePlus can be worn round-the-clock, maximizing data collection, and delivering accurate results by the Aria algorithm.
Why it matters
It is a privilege for everyone in the Empatica team to participate in the effort to combat COVID-19 together with the U.S. Army Medical Research and Development Command. USAMRDC arguably has the world’s best track record in sponsoring and fostering groundbreaking technologies. Their inspiration to intervene and act with this initiative brings into sharp focus the impact COVID-19 has had in our daily lives, as well as the need to work together to face the challenge. This is not only a technological race against time but also a civic responsibility in employing our experience against a threat to our health and economy.
Empatica is currently the only manufacturer of a medical device system that combines a wearable, dedicated app, and algorithm to provide a daily COVID-19 risk assessment to its users.
While Aria is undergoing validation, we are accepting requests for pilots. Reach out to our team through this form or email research@empatica.com: we’ll be happy to explain more about how Aria works, and to identify if the profile of your organization fits the requirements. You can also learn more by visiting our dedicated webpage.