How do I choose between a wearable medical device and smartwatch for seizure monitoring?
In recent years, the line between fitness tracking, lifestyle accessories and medical device solutions began to blur.
Consumer smartwatches have been moving into the health monitoring space, and the design of medical devices has become more consumer-friendly.
This is an exciting advancement, but it also makes knowing which to choose and what the difference is hard. It can be difficult to know if you’re getting the most reliable solution, especially if it is for a medical condition, like epilepsy.
If you live with or care for someone who experiences tonic-clonic seizures, you may be looking for a seizure alert device that detects seizures, sends seizure alerts to caregivers, and monitors epilepsy. But you may not know how to distinguish the most trusted seizure detection watches from others.
How can I tell the difference between a medical device and consumer smartwatch? Are there any associated risks with those without the FDA clearance? How can I tell if the detection will work as expected and if it is a reliable choice? These are all common questions.
But don’t worry, we’re here to help!
One important way to make the distinction is by seeking out solutions cleared by the FDA.
The FDA clearance is important as it identifies that the wearable medical device in question is safe and reliable for a specific purpose, having been rigorously tested.
Choosing an FDA-cleared wearable is similar to choosing approved medication, or flying in a plane that has been designed with the highest standards in mind. In all of these cases, you’re choosing to trust something that is reliable and safe for the specific purpose it is intended for.
FDA-cleared seizure monitoring and detection devices
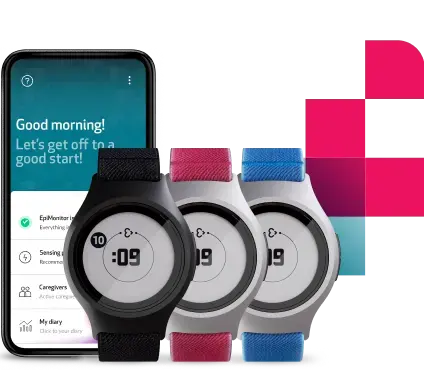
Empatica is proud to have made the only FDA-cleared watch for seizure detection available in the US. EpiMonitor is an all-in-one wearable and smartphone app solution designed and validated to detect patterns associated with possible tonic-clonic seizures, alert caregivers during emergencies, and monitor epilepsy.
But what does the FDA clearance mean and why is it important?
When a product receives FDA clearance, it means that it went through a 510(k) premarket submission, and the U.S. Food and Drug Administration (FDA) has carried out a robust review of the product’s effectiveness and risks, and deemed it safe and effective for its intended use before it can be sold in the US.
Let’s look at some FDA definitions of the 510(k) clearance, which EpiMonitor received.
“Generally, the FDA “clears” moderate-risk medical devices (Class II) (for example dialysis equipment and many types of catheters) for marketing once it has been demonstrated that the device is substantially equivalent to a legally marketed predicate device that does not require premarket approval.
Class II devices are generally subject to special controls, which may include specific testing or labeling requirements for that device.” [1]
So if you’re on the lookout for a new medical device solution to monitor your epilepsy, such as EpiMonitor, ensuring that it has been FDA-cleared confirms the testing process it has been through, its safety, reliability, and that the clearance is for the features you intend to use.
Benefits of FDA-cleared wearables vs consumer-grade smartwatches and fitness trackers
The clearance process for medical devices involves rigorous testing carried out independently in the lab, to ensure that the results are accurate, it is user-friendly, and that the data is secure.
- Safety and reliability: As there are strict controls in place for wearables with FDA clearance, there are likely to be fewer risks associated with them compared to consumer wearables. Given consumer wearables have lower safety standards, they have the potential to cause injury or harm, or be ineffective. But those that are FDA-cleared have high standards of safety, and can be trusted to be safely worn continuously.
- High quality: Due to the extensive testing, FDA-cleared devices are more reliable than consumer wearables and should last longer. This should be more cost-effective too, as users do not need to replace their device as frequently, nor do they need to worry about the device malfunctioning when they might need it to call for help.
- Reimbursement opportunities: As FDA-cleared devices follow stringent controls and have fewer risks compared to consumer wearables, the chance of reimbursement from your insurance provider may increase. This helps to make wearable medical devices accessible to more users. You may be able to get EpiMonitor reimbursed through your insurance. This depends on your specific plan and coverage level. Plus, if you live in Minnesota, under a new legislation medical devices and equipment like seizure detection devices will be covered by medical assistance.
- Reliable information for you and your doctors: FDA-cleared wearables work with clinically validated algorithms, meaning the algorithms that monitor your health are developed and trained using large data volumes and in a clinical setting. This means they provide more reliable and accurate data, which doctors are more likely to trust than that of consumer watches. This means that you can use your data to have insight-led conversations with your doctor, to ensure you’re on a treatment plan that works best for you.
Always check that the device is FDA-cleared for the usage you intend to use it for.
It is important to remember that some consumer smartwatches are also FDA-cleared. However, this does not mean such a device is necessarily the right or best choice for your epilepsy monitoring.
A wearable can receive FDA clearance for various purposes, but the clearance may be for features that are not related to your epilepsy. This means that the features that led you to make the purchase in the first place may not be any safer or reliable than a consumer wearable without an FDA clearance.
EpiMonitor is the only FDA-cleared wrist-worn wearable in the US for epilepsy monitoring.
The FDA-cleared EpiMonitor has been designed to give users and their caregivers even more peace of mind, by providing them with a solution they can trust.
In the words of Empatica’s Chief Scientist and Co-Founder, Dr. Rosalind Picard, “EpiMonitor is the device we’ve long dreamed of to open a new era in giving epilepsy patients greater control over their lives”.
EpiMonitor has a 98% seizure detection accuracy against the gold standard of hospital-based EEG measurement, designed specifically to get you help during a seizure.
It has been FDA-cleared for use in adult and children populations aged 6 and up, and is the only wearable solution with regulatory approval for tonic-clonic seizure detection available to purchase in the US.
EpiMonitor’s key features
- FDA-cleared for adults and children ages 6 and up
- Automatic tonic-clonic seizure detection through a smart algorithm (98% accuracy; low False Alarm Rate) and alerts to caregivers via a call and SMS with the location of the user
- Up to 7 days of battery life
- Advanced sensor technology for accurate data collection
- Ability for users to send self-triggered alerts to caregivers
- All EpiMonitor users based in the US will have the opportunity to participate in Empatica’s groundbreaking seizure forecasting study
- A user-friendly mobile app with a seizure diary
- Sleep and activity tracking through Empatica’s algorithms
- Seizure, sleep and activity reports that can be exported and shared with a physician
- Compatibility with iOS and Android smartphones
At Empatica, our focus is on making medical devices and algorithms for health monitoring that can be part of daily life. We are proud to have received FDA clearance for EpiMonitor, and we hope it will continue giving extra peace of mind to our users and their loved ones. If you would like to learn more about EpiMonitor and how it works you can visit our dedicated page.
- https://www.fda.gov/consumers/consumer-updates/it-really-fda-approved#:~:text=The%20FDA%20approves%20new%20human%20drugs%20and%20biological%20products.&text=This%20means%20that%20a%20company,product%20to%20federal%20quality%20standards.
- https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device
- https://www.fda.gov/medical-devices/consumers-medical-devices/are-there-fda-registered-or-fda-certified-medical-devices-how-do-i-know-what-fda-approved
We do not guarantee that EpiMonitor will detect every single seizure and deliver alerts accordingly. It is not meant to substitute your current seizure monitoring practices, but rather to serve as a supplement in expediting first-response time.