The Empatica Health Monitoring Platform receives FDA-clearance
We are happy to announce the clearance of the Empatica Health Monitoring Platform by the U.S. Food and Drug Administration (FDA).
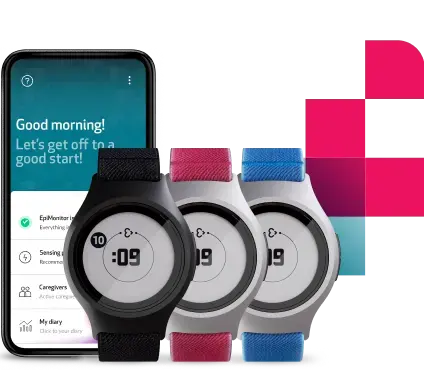
The Empatica Health Monitoring Platform is our full-stack remote health monitoring and data collection solution for research and healthcare professionals, built on data collected by our medical-grade and CE-certified EmbracePlus wearable. In addition to EmbracePlus, the Platform includes Empatica’s proprietary Care software suite, secure cloud infrastructure, and clinically validated digital biomarkers.
The clearance provides healthcare professionals and researchers with a modern, intuitive and reliable tool, and clinically validated digital biomarkers to remotely collect data that can be used to monitor Electrodermal Activity (EDA), SpO2, skin temperature and movement during sleep.
How the Empatica Health Monitoring Platform works
The Empatica Health Monitoring Platform is designed to ensure the continuous, seamless collection of physiological data from a diverse set of patients and clinical trial participants. All collected data can be easily accessed through the Empatica Cloud, while the Care Portal enables easy study management and patient onboarding.
Data is automatically collected by the EmbracePlus sensors and sent to the Care App via Bluetooth. The app then transfers this data to the Empatica Cloud using the phone’s internet connection, from where it can either be visualized through the Care Portal or downloaded for further analysis, both in raw format and as digital biomarkers.
The only FDA-cleared solution enabling continuous collection of SpO2, EDA, skin temperature, and movement during sleep
Empatica’s digital biomarkers are based on trained algorithms that analyze sensor data in one-minute intervals. The Empatica Platform has been cleared for continuous data collection to monitor blood oxygen saturation (SpO2) during rest, peripheral skin temperature, activity associated with movement during sleep, and Electrodermal Activity (EDA). All algorithms were validated against gold standards in clinical studies conducted with diverse participant groups. If you are interested in reading more about the studies we conducted and the results, simply reach out to our team who will be happy to share our validation work with you.
In addition to the measures included in the clearance, users of the Platform also have access to raw data continuously collected by the EmbracePlus’ EDA, PPG, Temperature, Accelerometer and Gyroscope sensors, and additional exploratory digital biomarkers such as Pulse Rate, Pulse Rate Variability and Respiratory Rate.
Opening up new possibilities for novel therapeutics and digital biomarker development
Over recent years, increasing numbers of pharmaceutical and biotechnology companies have shifted to hybrid and decentralized clinical trial models, augmenting site-based data collection with sensor-derived data collected in home and ambulatory settings. The Empatica Health Monitoring Platform is already being used globally in dozens of hybrid and decentralized clinical trials to continuously gather and analyze physiological data for research studies evaluating the impact of novel therapeutics. Using the Software Development Kit in the Health Monitoring Platform, researchers are also developing additional digital biomarkers to be used as endpoints in clinical trials.
Beyond clinical trials, the Empatica Health Monitoring Platform offers an array of exploratory and research-grade digital biomarkers, making it an ideal solution for academic research and longitudinal studies.
Do you have more questions about the Empatica Health Monitoring Platform and how your research and trials can benefit from it? Reach out to our team by filling out a request form or by writing to sales@empatica.com.