2025: Empatica’s year in review
2025 was a year of steady momentum for our team, one defined by meaningful product evolution, deeper engagement with the clinical research community, and important steps forward in how digital health technologies (DHTs) are applied.
Over the course of the year, Empatica managed a growing and operationally active clinical research portfolio across multiple therapeutic areas and geographies. We witnessed an increasing need for DHTs in trials, which translated into large-scale device deployments. Our technologies were used across the clinical development spectrum, from pilot through late-phase research, reflecting increasing confidence in wearable-based digital endpoints across a diverse set of therapeutic areas, with active trials in neurology, endocrinology, respiratory, mental health, cardiology, and more.
Throughout the year, we continued providing our technology for objective, at-home neurology monitoring, supporting thousands of people living with epilepsy, while new innovations and milestones meant our validated DHTs have come closer to broader use in both clinical research and care.
Together, these developments set the foundation for the moments that shaped the year.
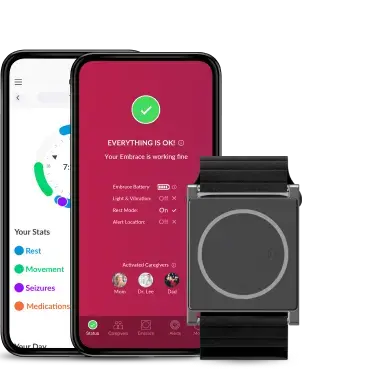
Growing Empatica’s potential through the strategic acquisition of PKG Health to expand into clinical care
A defining moment of the year came with the announcement of our acquisition of PKG Health, marking a significant step forward in advancing objective Parkinson’s disease monitoring. By bringing together PKG’s validated algorithms with our wearable technology, we strengthened our ability to support both research and clinical care in movement disorders. We look forward to sharing some important developments on this front in the new year.
Enhancing the DHT capabilities of the Empatica Health Monitoring Platform with EmbraceMini and eCOA
When it comes to wearable devices for clinical trials, DHTs need to evolve alongside the needs of the sponsors and the participants. In 2025, we worked closely with our clients to understand how to address the growing demand for actigraphy solutions optimized for low participant burden and scalability across diverse study designs. That is why one of our biggest highlights of the year was the launch of EmbraceMini, our smallest wearable to date, designed specifically for actigraphy.
EmbraceMini is a slim wristband made to support clinical trials with low participant burden and high-quality data collection. We took a deeper look at the device’s capabilities and its role in supporting continuous, real-world data collection in our June webinar, which you can watch here. With EmbraceMini, we are putting the participant at the heart of clinical trials.
Our vision is to provide a single platform for diverse health monitoring needs, reducing the need for multiple tools and, thus, reducing the burden on patients and sites. That is why we are continuously expanding the data-collection capabilities of the Empatica Health Monitoring Platform. By building an eCOA functionality directly into the Care App, we are enabling functional assessments and patient-reported data to be captured alongside continuous physiological measures within a single, regulatory-grade system.
We also introduced multi-device support using two or more devices (both EmbracePlus and EmbraceMini), expanding flexibility for study designs that require simultaneous data capture across multiple body locations or use cases.
In June, we achieved a landmark regulatory milestone for our Platform: the U.S. Food & Drug Administration has authorized Empatica’s pulse oximetry (SpO2) algorithm with a Predetermined Change Control Plan (PCCP), making it the first ever PCCP authorization for a pulse oximetry device.
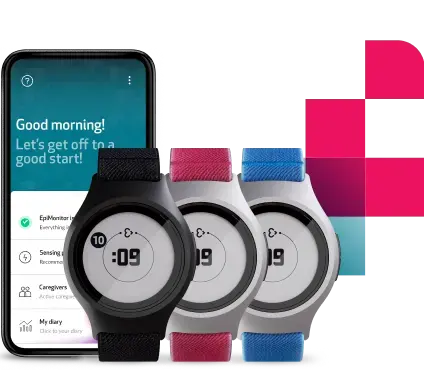
Advancing epilepsy monitoring and bringing EpiMonitor to more people with epilepsy globally
EpiMonitor became available for purchase in the EU, expanding access for people living with epilepsy and their caregivers. This was thanks to the receipt of the Medical Device Regulation certificate, a significant regulatory milestone that reinforced EpiMonitor’s readiness for broader clinical use in Europe, as well as our products’ standards of quality and performance.
The European launch coincided with the release of new bands for EmbracePlus, introducing all new color variations and continuing to refine the device experience, allowing people living with epilepsy to better personalize EpiMonitor to match their personal style.
Engaging with the global clinical and neurology communities
Throughout 2025, Empatica maintained an active presence across the global clinical research community, using in-person engagement to advance dialogue around real-world data, digital endpoints, and scalable trial execution. In parallel, we continued to generate and share scientific evidence through conference presentations and collaborations, reinforcing trust in wearable-based digital biomarkers as reliable tools for both research and care.
Across these engagements, one theme consistently emerged: the growing expectation that wearable data be both scalable and clinically meaningful.
Advancing dialogue across modern trial design and digital endpoints
The year began with the 16th Annual SCOPE Summit in February, where discussions focused on how wearable technologies and digital biomarkers can support more efficient, patient-centric clinical trials. In March, we continued these at a Merck-hosted event, engaging industry partners on topics including digital endpoints, data quality, and the evolving role of wearables in clinical development.
Momentum continued through the second quarter as we engaged directly with clinical research partners at events including the Digital Breakthrough for Pharma Summit (BMS) in June, and a Roche-hosted event in Basel. Across these forums, discussions explored how digital biomarkers and wearable-based monitoring can be integrated into modern trial designs to improve data quality, operational efficiency, and participant experience. In September, engagement continued at DPHARM, where conversations focused on innovation in clinical trials and the role of digital tools in advancing both data quality and patient experience.
Deepening engagement across neurology and movement disorders
The final quarter of the year was defined by engagement with clinical research, movement disorders, and digital health communities worldwide. We participated in the Digital Implementation Summit, hosted at Pfizer and co-created with Johnson & Johnson Innovative Medicine, where our CEO and Co-founder, Matteo Lai, shared perspectives on Parkinson’s monitoring following the acquisition of PKG Health.
Throughout this period, we engaged closely with the movement disorders community at events including the International Parkinson and Movement Disorder Society (MDS) Congress, the Swedish Movement Disorder Society Annual Meeting in Uppsala, and the Scottish Association of Neurology (SANS) meeting. These discussions focused on how objective, wearable-based measurement can support more consistent, real-world insights in Parkinson’s research and clinical care.
Continuing commitment to epilepsy research and community engagement
Engagement in epilepsy monitoring, research, and advocacy remained central across 2025, but the culmination of these was joining the community in Atlanta for the Partners Against Mortality in Epilepsy (PAME) Conference and the American Epilepsy Society (AES) Annual Meeting, sharing real-world evidence, supporting SUDEP awareness, and continuing conversations on the role of continuous monitoring in improving outcomes.
We also participated in Epilepsy Awareness Day at Disneyland Resort, presented by Sofie’s Journey, where we connected directly with people living with epilepsy, caregivers, and clinicians, at an event highlighting the importance of education, advocacy, and community in advancing epilepsy care. Our commitment was further reinforced through participation in the 36th International Epilepsy Congress in Lisbon.
Closing the year with forward-looking perspectives
2025 concluded with continued Parkinson’s-focused engagement at the Parkinson Study Group Annual Meeting in San Diego and the International Parkinson and Movement Disorder Society Wearable Systems Workshop in Madrid. Discussions at these events explored emerging technologies, AI applications, and the future of movement disorder monitoring.
Looking ahead
As we reflect on 2025, one theme stands out: progress built through collaboration, across disciplines, therapeutic areas, and geographies. From product innovation and regulatory milestones to research partnerships and global engagement, the year reinforced our commitment to enabling objective, real-world insights that can meaningfully support clinical research and care.
Beyond research, these efforts are ultimately aimed at a broader goal: improving patient outcomes and supporting better health for more people through reliable, continuous measurement in real-world settings.
We’re grateful to the researchers, partners, and community members who contributed to this journey, as well as to our team for the work behind the scenes that made this progress possible. We look forward to continuing this work in the year ahead.
To continue following how these developments translate into real-world research and care, follow our LinkedIn page and subscribe to our newsletter for insights, updates, and upcoming milestones.