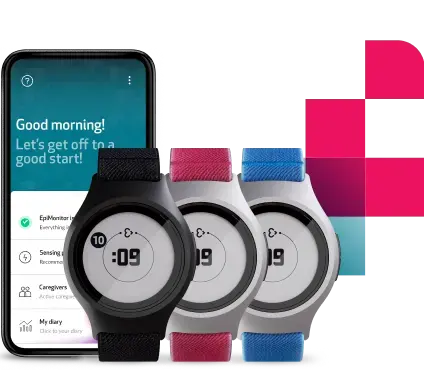
2
data collection sites
At King’s College London and the Vall d'Hebron Research Institute (VHIR) in Barcelona
300
participants 18+
Participants selected from ADHD clinic waiting lists
12
months wear time
Participants asked to wear EmbracePlus continuously for 12 months
“We are thrilled to be working with our SME partner, Empatica, to incorporate their new EmbracePlus device into the ART system, to obtain unobtrusive, real-time, detailed data on, physical activity, sleep, autonomic arousal, and indicators of cardiovascular health (heart rate per minute, heart rate variability, oxygen saturation (SpO2)).”
Hayley Denyer, Ph.D. student at King’s College London
ADHD is diagnosed based on impairing levels of inattentive, hyperactive, and impulsive behaviors. It is a common psychiatric disorder, with a 2.5% prevalence among adults[1]. Most adults with ADHD present with additional serious mental health problems that can include anxiety, depression, emotional instability, antisocial behavior, substance use, and autism spectrum disorder[2]. Emerging evidence further points at substantial comorbidity and shared genetics between adult ADHD and cardiometabolic diseases (obesity, type-2 diabetes, and cardiovascular disease). However, detailed knowledge about the screening, diagnosis, and clinical management of adults with ADHD and co-occurring cardiometabolic disease is lacking.
Pharmacological treatment for ADHD includes both stimulant and non-stimulant medications. Randomized control trials (RCTs) have shown short-term beneficial effects of ADHD medication on the core symptoms of ADHD; yet estimated treatment non-adherence (not taking medication as prescribed) ranges from 13.2% to 64% in individuals with ADHD[3, 4]. One reason for treatment non-adherence is due to unwanted treatment side-effects, including increased heart rate and blood pressure, and changes in sleep and eating behaviors [5]; yet long-term real-world data is lacking for treating non-adherence in adults with ADHD.
To address these gaps in research, real-world data from patients’ daily lives is needed – and this is where ART-CARMA comes in!
ART-CARMA
Our team – led by Professors Jonna Kuntsi and Richard Dobson – has recently developed and piloted a new ADHD Remote Technology (‘ART’) system. The ART system, which benefits from an existing ‘RADAR-base’ mobile-health platform, allows for real-world remote data collection on the clinical symptoms of ADHD and co-occurring disorders, functional and cognitive impairments, and health behaviors (such as exercise and sleep) on large sample sizes. Our new study,
A
DHDR
emoteT
echnology study ofca
rdiometabolicr
isk factors andm
edicationa
dherence (‘ART-CARMA’), utilizes the ART system and incorporates elements of active monitoring (questionnaires, cognitive tasks, speech samples) and passive monitoring (smartphone passive app and a wearable device). ART-CARMA is a clinical study that forms part of TIMESPAN, a 5-year-long, collaborative and multidisciplinary, European Commission-funded research program with 17 partner institutions across Europe and the world, including academia, small and medium enterprises (SMEs), patients, and care providers. There are two data collection sites in the ART-CARMA project: in addition to the team at King’s in London, a second data collection team is based at Vall d'Hebron Research Institute (VHIR) in Barcelona, led by Professor Toni Ramos-Quiroga.We are thrilled to be working with our SME partner, Empatica, to incorporate their new EmbracePlus device into the ART system, to obtain unobtrusive, real-time, detailed data on, physical activity, sleep, autonomic arousal, and indicators of cardiovascular health (heart rate per minute, heart rate variability, oxygen saturation (SpO2)).
Across the two data collection sites, we will be recruiting 300 adults (aged 18+) from adult ADHD clinic waiting lists. Participants will be asked to wear the EmbracePlus continuously for 12 months. They will also be asked to download a smartphone passive app on their smartphone and answer a range of questionnaires at different intervals including (but not limited to) their symptoms and impairments of ADHD and co-occurring disorders, medication use and side effects, and to complete cognitive and speech tasks. What makes the study special is that we are monitoring individuals using the EmbracePlus and the other measures from an initial period of pre-treatment, through to treatment initiation and titration, and beyond – for 12 months in total. We will be able to identify changes to objectively collected measures during naturalistic periods on- and off-ADHD medication treatment.
The EmbracePlus is an advanced wrist-worn health monitor and will collect important outcome measures to address our aims. The EmbracePlus will obtain real-time data on heart rate and heart rate variability, inter-beat interval, electrodermal activity, oxygen saturation (SpO2), peripheral temperature, and acceleration. By combining data across these variables measured, processes such as physical activity, sleep, autonomic arousal, and indicators of cardiovascular health can be captured.
The EmbracePlus will help us to:
Identify real-world consequences of ADHD medication treatment on the following cardiometabolic risk (or protective) factors in adults with ADHD: including changes in heart rate, blood pressure, sleep, and physiological stress response.
Measure the potential contribution of physical activity to a reduction in these risk factors, independently and in combination with ADHD medication treatment.
Identify potential reasons for non-adherence, focussing on adverse side effects such as changes in sleep, heart rate, heart rate variability, inter-beat interval, electrodermal activity, and peripheral temperature.
For my Ph.D., I am particularly interested in utilizing EmbracePlus’ sensor suite and benefit from the sensor suite’s sleep analyses to look further at how sleep in adults with ADHD may change across the 12-month remote monitoring period, including periods on- and off-ADHD medication, and incorporating treatment initiation and titration. The EmbracePlus’ sensor suite reports on sleep and wake timing, turns and tosses, sleep efficiency and fragmentation, activity level over the day, and the features of EDA during wake and sleep.
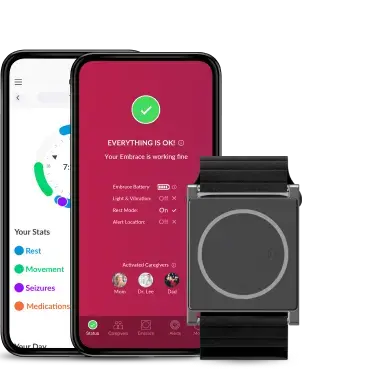
In our testing of the EmbracePlus, we have found it comfortable and stylish, and the Care App that participants download onto their phone is easy to use. The use of the color-coded system on the Care App will allow participants to know instantly if there are any connectivity issues between their EmbracePlus and phone and will be helpful during data collection. Whilst testing the devices here at King’s, we have further found that the EmbracePlus battery lasts a few days and doesn’t take too long to fully charge again. This means participants can also charge their devices whilst they shower and get ready and continue to wear it for the rest of the day - meaning fewer gaps in the data. The Care App also has a handy reminder to let you know when the battery is low, which I’m sure will be useful for our participants.
As a researcher, having access to Empatica Care is going to be valuable during data collection. Empatica Care provides adherence information that will allow us to actively monitor whether a participant is or is not wearing the device correctly, or if the EmbracePlus is not connected to the smartphone. We can then reach out to the participant during their 12-month study period to provide support and to understand if there are any charging or connection issues, or why they may not be wearing the device.
Our hopes for the future
We hope that ART-CARMA will improve the management of cardiometabolic disease and improve ADHD medication treatment adherence in adults with ADHD.
Credit
This project has received funding from the European Commission Horizon 2020 research and innovation program under grant agreement No. 965381. Hayley Denyer is supported by the UK Medical Research Council (MR/N013700/1) and King’s College London member of the MRC Doctoral Training Partnership in Biomedical Sciences.
***Hayley Denyer is a Ph.D. student at the Social, Genetic, and Developmental Psychiatry Centre at the Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, funded by the Medical Research Council Doctoral Training Partnership. Hayley’s Ph.D. focuses on the application of remote measurement technology to identify health behaviors and targets for intervention in individuals with attention deficit hyperactivity disorder (ADHD). Hayley has been involved in the ADHD Remote Technology (ART) research program since 2019 and is the study coordinator of the ART-CARMA study.@hayleydenyer_