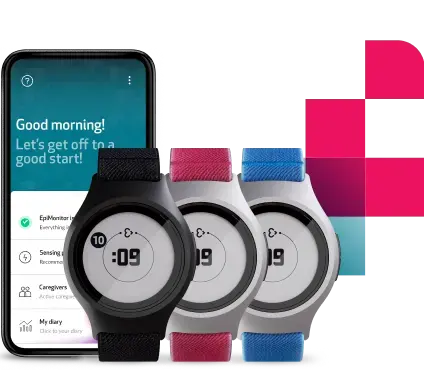
Achieving scalability in clinical research with EmbracePlus
EmbracePlus will be released later this year and, will be offering the world’s most mobile, flexible, and accurate clinical platform in Neurology research. It has been designed and developed to suit even the most advanced type of research needs, incorporating the best of Empatica’s existing research solutions and our consumer-focused, FDA-cleared monitor, the Embrace2.
Although EmbracePlus for Research will offer clinical investigators around the world multiple benefits, we would like to focus on scalability as the specific subject of this post.
In today's economy, the globalization of clinical research provides great opportunities for significantly improving the efficiency of the drug development process, with faster timelines and increased patient populations. Global trials, however, pose daunting challenges to many other major aspects of clinical trials, including but not limited to, design, analysis, ethics, law, and regulation [1-2].
What are the common challenges investigators face when conducting a large-scale clinical trial and how can the EmbracePlus for Research help overcome them?
Complexity
Clinical trials have become increasingly complex over the years, and for big pharma and small biotech alike, the aim is to design trials that give the right answers, in the most simple and uninterrupted unobtrusive way for patients [3]. With a modern design and a combination of powerful sensors, EmbracePlus enables the uninterrupted monitoring of patients or research subjects. It continuously collects and sends raw physiological and computed data via Bluetooth to the paired app.
Through the Research Portal (a cloud platform), research teams, CRO personnel, and study coordinators can access the continuously uploaded and protected sensor data, lead investigations, check compliance, and manage multiple sites.
The EmbracePlus for Research can reduce the complexity of global trials not only with a simplified technological ecosystem but also with the flexibility of its components. Empatica’s SDK allows researchers to pull data into their own platform, and the Research Portal is configurable to match individual companies’ workflows and preferences.
Geographical Diversity
Geographical diversity can be an important element of data collection to ensure the results of a trial are robust and take into account factors that may otherwise be overlooked by a homogeneous data set. Clinical trials across multiple regions of the world have become common practice [4]. According to the National Institutes of Health, a division of the U.S. Department of Health and Human Services, there are currently more than 330,00 trials taking place in more than 200 countries.
Diversity in trials comes with multiple challenges.
At a logistical level, organizations implementing a global clinical trial program need to invest time and resources to ensure that the countries they enter have the infrastructure to support the trial [5]. This means not only enough safe, sanitary facilities, but also access to these facilities. The lack of infrastructure can often result in lost traceability, transparency, and control of the data. At the analysis stage, due to the heterogeneity in many trial aspects, the interpretations of the results for a global trial can be markedly more difficult than those for a single-region trial with a homogeneous patient population.
EmbracePlus has been designed to overcome geographical boundaries to help researchers collect a diverse, objective, and accurate data set with minimum friction. It runs on the same FDA-cleared, robust, mobile, and cloud data pipeline infrastructure as Embrace, enabling the management of thousands of subjects and seamless data transfer. Its objective data collection through sophisticated sensors overcomes the language barriers that could be presented by paper diaries.
Through the Research Portal, study coordinators can visualize data from thousands of devices in real-time, enabling at-a-glance analysis and efficient management of multi-country clinical trials.
Technology adoption
In site-less trials, the patients become responsible for receiving the investigational product, completing study assessments, troubleshooting equipment or technology problems, etc. This can be a burden for some of them, particularly in older populations and those who are not technologically savvy [6]. Learning how to use the technology can be time-consuming.
Collecting data for each participant in a clinical trial efficiently and accurately and according to the study, objectives is essential for regulatory compliance, as well as the success of the research effort.
The EmbracePlus features an interactive interface, complemented by the in-app onboarding process with instructions on its functionalities and comprehensive, quick start guides. The device has also been designed to be appealing and comfortable to wear 24/7: it’s extremely light, water-resistant, and has a modern and captivating design. Its easy-to-use and easy-to-wear properties ensure higher rates of subjects’ enrollment, engagement, and compliance.
Costs
Clinical trial costs can vary widely depending on the number of patients and sites (including full staff and facilities) and the complexity of the trial protocol [7]. One of the most expensive components of a clinical trial is monitoring which can account for anywhere between 25% and 30% of the overall cost of a research program [8].
The patient-centric model of the EmbracePlus for Research shifts the responsibility of data collection to the study participants, rather than the research site. By enabling the continuous, passive, and unobtrusive monitoring of the study subjects, the EmbracePlus model reduces the need to activate and monitor sites, negotiate contracts, and pay stipends. It also minimizes office visits, physician-administered procedures, and error-prone data entry.
Through the Research Portal, research teams, CRO personnel, and study coordinators can understand at a glance if and how often enrolled subjects are wearing the device correctly throughout the study. This allows for spotting and quickly solving low-compliance issues. They can also rely on fast, secure data transfer and analysis, maximizing the quality and quantity of data collected.
The technology behind our Embrace system is trusted by thousands of partners (NEC and Sunovion to name a few), to empower their investigations. Our years of experience with multiple pharmaceutical companies in aligning with strict privacy, security, and IRB regulations also allow us to provide our clients with the required documentation, considerably reducing their time and resources over regulatory and administrative barriers.
Become an early adopter
Did you know? The EmbracePlus has been chosen by the Translational Research Institute for Space Health (TRISH), in partnership with NASA’s Human Research Program (HRP), to be the first wearable to monitor the health of the astronaut crew that will be on board the first manned mission to Mars!
EmbracePlus will be available later in the year, but early access is available to select clients. Get in touch with our team here to have all your questions answered!
Those of you conducting smaller-scale trials that require real-time data analysis can take advantage of our offer for early adopters and get two medical-grade devices to power your studies in one go: buy an E4 wristband today and become eligible for a discounted upgrade to EmbracePlus upon its release.
Find out more about how E4 can empower your research via real-time physiological data acquisition and visualization or purchase it now to take advantage of the bundle offer.
Words worth reading
Sources:
- https://pharmaphorum.com/views-and-analysis/challengesandopportunitiesinglobalclinicaltrials/
- https://www.researchgate.net/publication/262798491TheOpportunitiesandchallengesinconductingclinicaltrials_globally
- https://informaconnect.com/report-biggest-challenges-clinical-trials-pt-1/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4840793/
- https://www.mddionline.com/clinical-trials-go-global
- https://www.clinicalleader.com/doc/the-benefits-and-challenges-of-siteless-clinical-trials-0001
- https://www.ncbi.nlm.nih.gov/books/NBK50888/
- https://www.pharmavoice.com/article/trial-cost-cutting/