How can Embrace2 for Research empower your study?
A new post dedicated to our researchers is here, and we’re thrilled to give you an in-depth view of Embrace2 for Research.
What is it? A reliable, non-invasive system, created for researchers by researchers, to facilitate real-world evidence (RWE) collection and multi-site trial management.
The ecosystem is twofold:
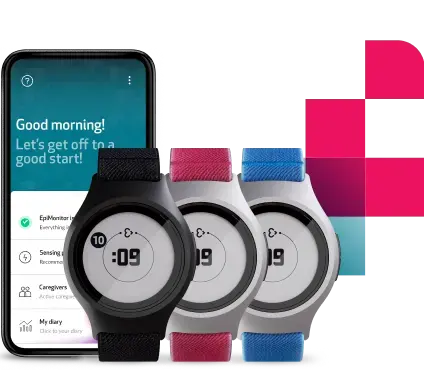
- The Embrace2 smart watch for study patients that continuously collects and sends raw physiological and computed data through Bluetooth to a paired app: Mate.
- The Research Portal for site coordinators, CROs, or researchers to access continuously uploaded and protected sensor data
What’s the impact in the research world?
- It eases the management, scalability, and complexity of site-focused or virtual trials.
- It guarantees objective, secure, and remote, real-world data gathering, without the need for physical visits at hospitals.
- It has been designed to speed up subject enrollment and increase their engagement, as they are only required to wear a minimal and stylish wristband to participate in the study.
- It may even accelerate the path to breakthroughs, thanks to an ecosystem optimized for continuous, clinical-quality observations in an almost real-time and readily visualized format.
The technology behind the Embrace2 for Research is trusted by thousands of partners to empower their investigations: among them are NEC and Sunovion. NEC is currently developing a technology that accurately estimates chronic stress based on the physiological information obtained by our wearable sensors, while Sunovion incorporated the Embrace2 into the trial design of its antiepileptic drug (AED) APTIOM® (eslicarbazepine acetate).
We also have partnered with DRIVe, a division of The U.S. Department of Health and Human Services, to work on a research project aimed to develop a new smart watch to alert users when they’re developing a respiratory infection, even before any symptoms appear.
Overview of each component of the ecosystem
The Embrace2
The Embrace2 is an FDA-cleared, wrist-worn, medical device that records physiological signals, but at the same time, works like any consumer wearable device, making data collection and subject compliance easier to manage.
Embrace2’s sensors continuously measure physiological parameters giving insights into sleep, activity, autonomic arousal, and seizures for many different typologies of clinical/research studies.
The device is designed to be comfortably worn 24/7 by the study subjects. It weighs only 13g, it is water-resistant for up to 1m/3ft, and it provides a 48h battery life with only a 90-minute charge. These features, together with the Embrace2’s minimal and sophisticated external design, contribute to overcoming the regular barriers that researchers and investigators face in subject recruitment, retention, and compliance.
The sensors monitor:
- Skin conductance: an output of sympathetic nervous system activation, it provides a measure of emotional arousal.
- Motion: measured by a 3-axis accelerometer sampled at 32Hz.
- Skin surface temperature: measured by a peripheral temperature sensor sampled at 1Hz.
You can learn more about the research behind Embrace’s technology, by referring to our Science page.
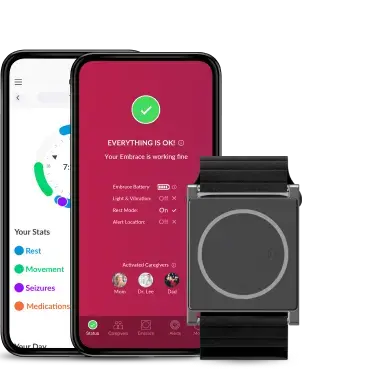
Embrace2 is also part of our epilepsy monitoring system, using an FDA-cleared algorithm developed to recognize unusual movements and physiological activity that indicates a possible tonic-clonic seizure. It offers seizure monitoring and alerting services for people living with epilepsy and their providers.
Benefit summary:
The Embrace2 guarantees clinically-validated data collection from a broad range of physiological signals and higher subject compliance, thanks to its comfortable and stylish design.

The Mate App
The Mate App is designed to gather physiological data, while also functioning as an e-diary. Subjects can mark relevant events as directed by the study, providing daily insights for understanding subjective outcomes.
Subjects log into the app with unique uploader credentials, generated by the Research Portal. Researchers can agree on a specific subject ID for each patient before starting the enrollment process. This is convenient as it de-identifies the research subjects so that no personal data is shared. The unique identifier also ensures that the subject's data is partitioned correctly and stored securely.
All physiological data is automatically uploaded to the Research Portal using the smartphone’s internet connection.
In turn, data is processed and can be sent back to the Mate App for visualization, so subjects can access an in-depth analysis of their rest and physical activity. This Rest is analyzed to reveal how fragmented and efficient the rest period was. Physical activity is arranged into intensity, duration, distance, and step count. The app can display all of this information in one place, making it easy to see the day at a glance and encourage subject retention and engagement even more.
Moreover, we’ve recently released a new version of the app, developed to offer a new design that is simple to understand, capable of crafting an engaging and meaningful experience for its users.
Benefit summary:
The Mate App provides secure and reliable data transfer and visualization. It keeps the subjects engaged with an intuitive e-diary that visualizes objective stats on daily rest and movement. Data is also qualitatively optimized since the subjects can manually add events/notes.

The Research Portal
The third tool of the system is the Research Portal, a cloud platform that allows research teams, CRO personnel, and study coordinators to manage investigations, check compliance, manage multiple sites, and collect data from different types of clinical and longitudinal research studies, all within one platform.
Whether your study is centered on seizures, autonomic arousal events, or generic physiological data acquisition, you can access and download raw sensor data and summary data directly from the Research Portal, along with all events manually added by the study’s subjects, which may provide important contextual information. This feature allows research teams to check the relevant raw data for patterns and begin analysis even as the study is ongoing.
We will delve deeper into the Research Portal’s features and benefits in our next [posts](), so stay tuned!
If you are interested in using Embrace2 and the Research Portal for your research study/clinical trial, you can get in touch with our team here.
Feel free to also visit our support page to learn more!
Words worth reading
We do not guarantee that EpiMonitor will detect every single seizure and deliver alerts accordingly. It is not meant to substitute your current seizure monitoring practices, but rather to serve as a supplement in expediting first-response time.