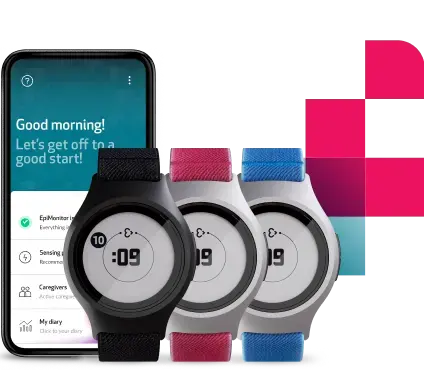
The EmbracePlus wearable wins European CE mark
After only a month from winning the CE mark for both Aria and Empatica Care, we’re pleased to announce that Empatica’s EmbracePlus wearable has received the European CE mark as a Class lla medical device, for its ability to consistently provide quality physiological parameters to its users.
Designed and engineered to be the world’s most powerful medical smartwatch, the EmbracePlus combines the best of Empatica’s existing wearables: our FDA-cleared Embrace2, and the E4 wristband used in hundreds of research studies. It continuously and remotely collects and processes key physiological signals from the wrist, including pulse rate, pulse rate variability, blood oxygenation, respiratory rate, skin temperature, electrodermal activity, rest, and actigraphy data. The data are continuously sent to a smartphone app and from there, they are transferred to the cloud for review and analysis by clinical researchers and healthcare professionals through a single dashboard. Data can be streamed simultaneously from thousands of devices. This is possible via Empatica software or via third party-applications that can be developed using our SDK.
Boasting Empatica’s proprietary PPG and EDA sensors, EmbracePlus is the only medically certified smartwatch that enables continuous vital signs monitoring alongside continuous EDA monitoring, an important metric in the field of neurology and psychiatry research. Beyond physiological data, EmbracePlus enables the development and monitoring of digital biomarkers, the output of data reprocessed by algorithms. An example of such a use case is Empatica Aria, which can detect the early onset of respiratory infections, including influenza and COVID-19.
EmbracePlus is built to enhance remote and passive data collection from clinical trial participants, which has become increasingly popular since the pandemic. Clinical trials depend on rich patient data, yet collecting such data has traditionally been expensive, time-consuming, and susceptible to error. Clinic visits only capture snapshots of a patient’s health, i.e., one electrocardiogram or phenotype analysis, while requiring patients to take time away from their work or families to visit a clinic. Wearables track patient data over large periods of time from patients’ homes, resulting in rich datasets. The combination, quality, and scale of data collected remotely by EmbracePlus can enable the creation of novel digital endpoints, allowing clinical trials to become patient-centric while uncovering new insights.
The select partners who already secured the technology
Among our most recent partners, Pear Therapeutics, the leader in prescription digital therapeutics (PDTs), will be using Embrace Plus’ sensors with the aim of evaluating withdrawal symptoms in patients with substance use disorder (SUD), opioid use disorder (OUD), and alcohol use disorder (AUD).
The clinical study ART-CARMA, part of TIMESPAN and led by a research team at King’s College London, will use the EmbracePlus to obtain real-time data from adults with Attention Deficit Hyperactivity Disorder (ADHD), with the long term aim of improving the management of cardiometabolic disease in adults with ADHD, and to improve ADHD medication treatment adherence and the personalization of treatment.
Another notable partnership is the one with the NASA-funded Translational Research Institute for Space Health (TRISH), which has chosen the EmbracePlus to be the first wearable to monitor the health of the astronaut crew that will be on board the first manned mission to Mars.
EmbracePlus is currently being rolled out for use with select clinical partners, reach out to our team through this form or email research@empatica.com to learn if the profile of your organization fits the requirements.
If not, you can still access Empatica’s technology to collect real-time physiological data safely and continuously with our CE-certified E4 wristband: the industry-standard wearable for experimental research, combining PPG and EDA sensors. With each purchase of an E4, you’ll be granted a free upgrade to the EmbracePlus once released. Don't miss out! Learn more by visiting our dedicated webpage.
We do not guarantee that EpiMonitor will detect every single seizure and deliver alerts accordingly. It is not meant to substitute your current seizure monitoring practices, but rather to serve as a supplement in expediting first-response time.