FDA-CLEARED
CE MDR CERTIFIED
Empatica Health Monitoring Platform for Clinical Trials
Empowering clinical research with high-precision digital measures

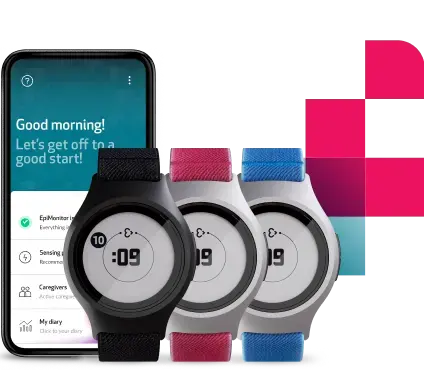
Enhance your clinical trials with raw data and digital biomarkers of the highest quality, with an easy-to-use, full-stack platform designed to reduce patient and site burden. Conducting research at scale from patients’ homes has never been easier.


Scale your research across studies and therapeutic areas with a single platform

Scalable
Scalable across studies and geographies, with simultaneous data collection from thousands of devices and multiple language support

Modular
One multi-use, adaptable wearable that can be worn across different areas of the body depending on your study needs

Reliable
Secure and compliant FDA-cleared technology and dozens of accurate digital measures, for guaranteed quality data


<2 months
from contract signing to starting data collection
>90% compliance
with EmbracePlus used as an actigraphy sensor¹
120+ Industry sponsored trials
have already implemented our Platform to collect data
1. Feasibility of implementing wearable technologies for in-home assessment of behavioral and event-related potential responses after sleep and wakefulness in Alzheimer disease
The only tool you need to remotely collect health data for your decentralized trials
Empatica Cloud
A full-stack, unified solution that’s built to work together
Cloud to Cloud
Use your own cloud while combining Empatica’s hardware and software for data collection and streaming. Discover Empatica's Cloud API


Designed for the patient
- All data is collected via Empatica’s medical-grade wearables, with a modular design, comfortable materials, waterproof capabilities, and extended battery life, designed to be worn in daily life.
- Data is streamed via a smartphone app that is simple to use and install on popular smartphone models. The app is translated into over 40 languages, with more continuously being added, allowing you to enhance diversity in your research without disadvantaging participants
- Setup is fast and easy, and can be done autonomously with the support of dedicated patient materials
- Battery life can last up to 28 days, reducing charging time.


Made to support global trial operations
- Remote and secure enrollment, participant, and study management through the Care Portal
- Data collection and streaming to the Cloud is continuous and automated, thanks to the EmbracePlus and EmbraceMini capabilities
- Continuous support by a dedicated team, help desk, and materials, covering site and patient needs across every step of your study
- Empatica’s experienced team handles all logistics, including device shipping, replacement, and returns
- Sites receive frequent adherence reports via email, so that staff can quickly intervene when they identify compliance issues
- Regulatory support and audit trails, including IRB/Ethics committee-friendly materials
- Empatica performs frequent testing and certification of new phone models, and provides provisioned phones, to ensure technological compatibility
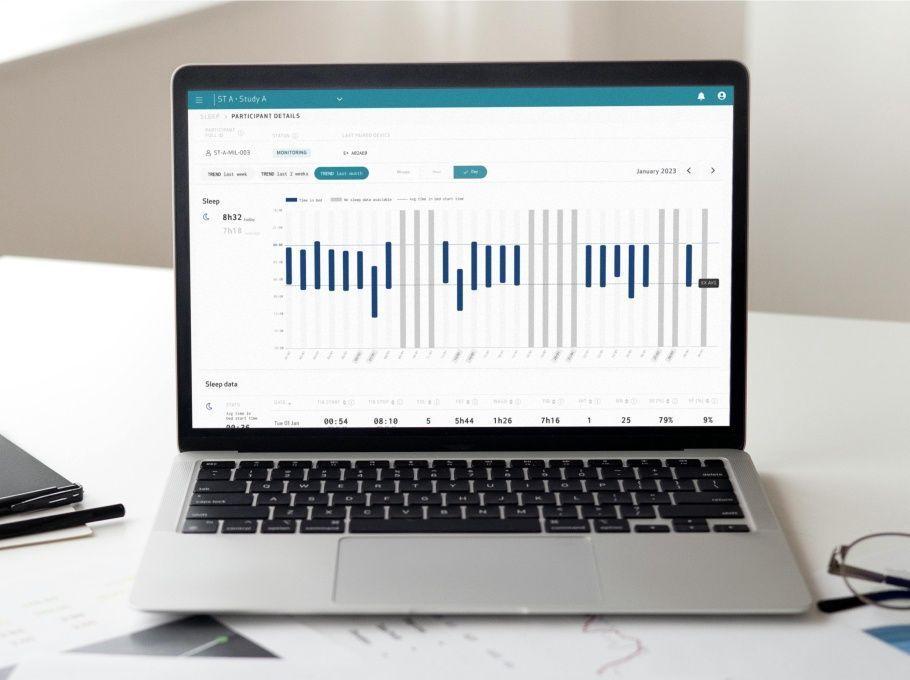

Enhanced for the scientist
- Purpose-made for remote data collection, the Empatica Health Monitoring Platform is FDA-cleared and developed to provide researchers with medical-grade data, collected continuously from participants’ homes
- Accelerate treatment research with over 300 clinically validated and research-grade digital biomarkers that can be used as digital endpoints in your trials
- Develop your own digital biomarkers using high-frequency raw data from the EmbracePlus sensors
- Visualize digital biomarkers and patient data inside the Care Portal, spotting patterns and adverse events




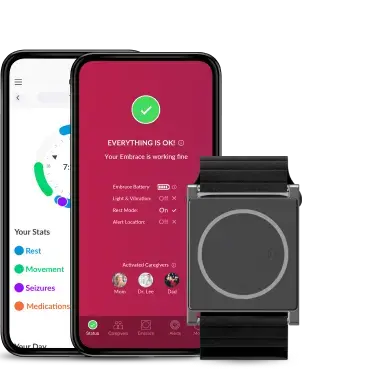
Powered by EmbracePlus
- The world’s most advanced and versatile medical device
- 5x powerful sensors: PPG, EDA, Skin Thermometer, Accelerometer and Gyroscope
- Fully adaptable to the needs of your study; balance power with battery consumption and data quality using preconfigured sensor modes, including Actigraphy modes
- Taking health monitoring beyond the wrist, EmbracePlus can be combined with different accessories such as a belt clip and an elastic strap to be worn across different areas of the body


EmbraceMini: The perfect actigraphy wearable
- Compact and discreet, ideal to be worn next to a favourite watch or accessory
- 4x sensors: Accelerometer, Gyroscope, Magnetometer, Ambient Light Sensor
- 14+ days of battery
- The smallest actigraphy device in clinical research
- Fully modular
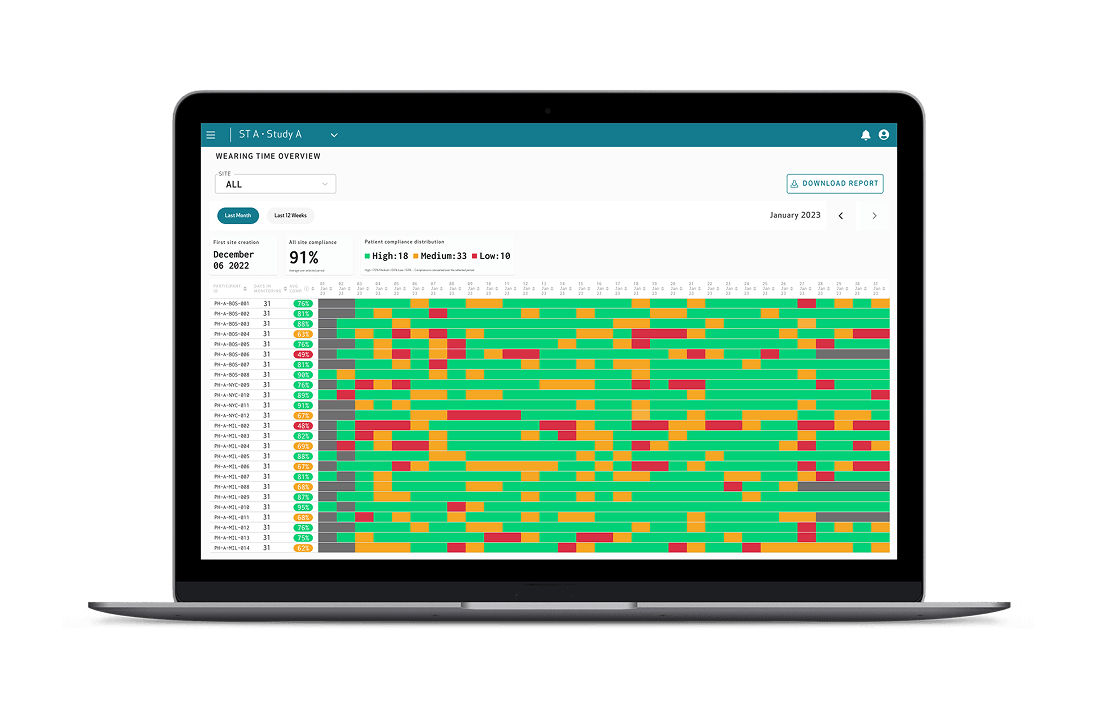
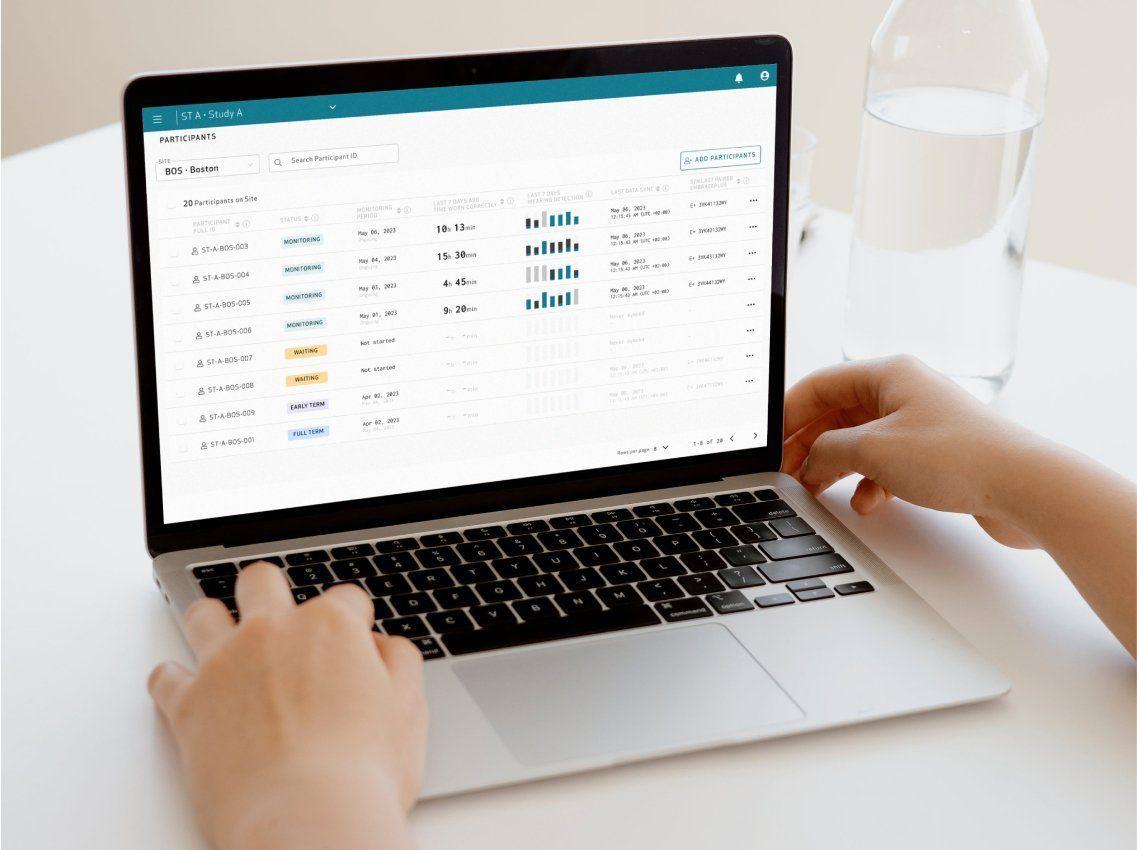
Seamless study management through the Care Portal
- Manage multiple studies and sites through a secure online portal
- Easily enroll and manage participants
- Visualize patient data and digital biomarkers across timeframes
- Wearing time reports and dashboards
Explore Care


The largest and most trusted selection of digital biomarkers in research
- Over 300 digital biomarkers, including dozens of sleep and activity measures
- 6 FDA-cleared measures
- Data visualization directly inside the Care Portal
- Algorithm integrations from trusted partners
Discover our range of digital biomarkers
Achieve multifaceted trial outcomes with double-device monitoring
Monitor each participant using 2x devices, in any form factor of your choice. Data flows into a single app installed on participants’ phones, ensuring the same, low-burden experience.
Track vitals and other specialized digital biomarkers alongside actigraphy data, with 300+ digital measures to choose from.
Reveal important correlations that single-device monitoring can miss with accurate and in-sync time-stamping.
Combine data collected from different body parts, such as lumbar and waist monitoring with wrist data.
Leverage the ability to capture different types of digital endpoints simultaneously, and demonstrate the reliability of your study.


Streamline clinical trials with Empatica’s Cloud API
Seamlessly integrate the Empatica Health Monitoring Platform into your existing clinical trial management system (CTMS) with our Cloud APIs.
- Reduce study complexities with seamless data flow
- Leverage Empatica’s 300+ digital measures and FDA-cleared medical wearable
- Manage EmbracePlus users directly from your own software
Learn more
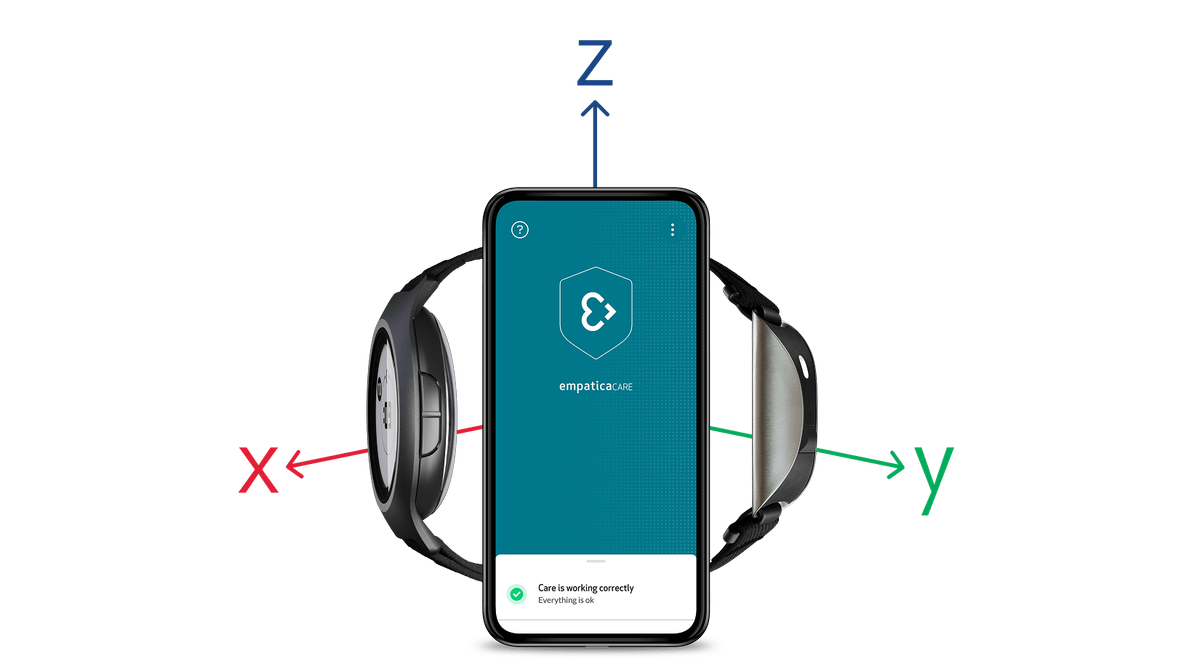
Empatica Actigraphy
Explore our full suite of activity-based digital biomarkers, available with our Actigraphy sensor modes
Learn more


Regulatory and Compliance
Empatica’s constant and decisive efforts aim at ensuring the safety, effectiveness, and quality of its medical devices as well as providing products that meet customer and applicable statutory and regulatory requirements
View Regulatory & Compliance
Thousands of papers have been published using our technology
We collaborate with the best research teams in the world to advance the understanding of human health in real-life settings. Our work is focused on measuring and quantifying the impact of wearable technology in health, while our products have been used in a breadth of contexts, from large-scale clinical trials to intimate academic studies.
Wondering if our solutions and digital biomarkers fit your needs?
Reach out and a member of our team will be in touch with you as soon as possible.