Empatica Secures CE MDR Certification for Parkinson’s Monitoring Using PKG Measures
Boston, MA — Empatica, a pioneer in digital biomarker development and patient monitoring using wearables and AI, today announced it has received CE Certification under the EU Medical Device Regulation (EU MDR 2017/745) for Parkinson’s monitoring via the Empatica Health Monitoring Platform, marking the launch of its Parkinson’s monitoring services for healthcare providers.
Empatica acquired PKG Health, a leader in movement disorder algorithms and digital endpoints for Parkinson’s disease, in October 2025. This milestone clears the Empatica Health Monitoring Platform and PKG’s validated movement disorder measures for clinical use in Europe and the UK, empowering healthcare teams to deliver more personalized, continuous, and data-driven care to millions of people living with Parkinson’s. The tools are also available for investigational use in the US, pending FDA clearance, which is expected later this year.
Parkinson’s is a progressive, lifelong, and currently incurable disease. Patients with Parkinson’s can manage their condition via periodic medication adjustments to maintain optimal symptom control. Yet, day-to-day symptom variation remains difficult to capture, and current Parkinson’s management relies heavily on patient recall, infrequent consultations, and subjective assessments.
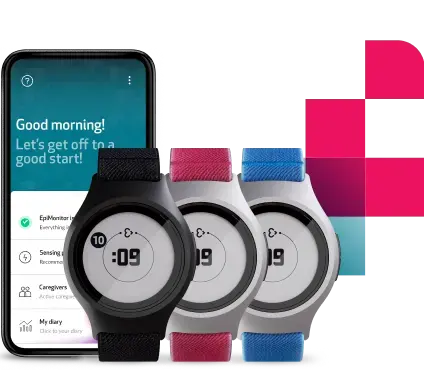
Empatica’s new Parkinson’s offering addresses this gap, combining PKG’s 38 motor assessment algorithms with the EmbraceMini wearable. Small, lightweight, waterproof, and with 14+ days of battery life, EmbraceMini is designed to deliver an enhanced patient experience alongside high-quality data, taken continuously in real-world settings and delivered inside the classic PKG report.
“This is a critical milestone for our hospital partners and patients,” said Matteo Lai, Empatica CEO and Co-founder. “They can experience the same results from PKG’s clinically validated algorithms, while leveraging the highly scalable Empatica platform and our EmbraceMini device. This means seamless monitoring, more targeted treatment adjustments, and better usability for patients. As a brand new product, we can't wait to receive users' feedback, and we have a lot more features coming up in the next months.”
A continuous view of key symptoms such as bradykinesia, dyskinesia, sleep scores, and other digital biomarkers can support evidence-based treatment decisions. Widely adopted in clinical practice, the PKG legacy system is supported by over 90 peer-reviewed publications. Evidence shows that 75% of the time, PKG insights influence a change in therapy, while 89% of patients say PKG data provides information they could not otherwise communicate, and clinicians note a 59% improvement in the quality of patient dialogue.
In a statement, Professor Per Odin, Head of the Division of Neurology at Lund University, said, “For years, the PKG has provided us with very valuable insights that we simply couldn’t capture during a consultation. Empatica’s integration of the PKG system and algorithms into its platform technology marks an exciting step forward for both patients and clinicians. It means we now have a solution that provides the same clinically-robust data, in real-time and at greater scale, with a significantly improved experience for everyone involved. It feels we are coming close to truly individualized, precision care for our patients.”
Healthcare providers can access data through Empatica’s cloud-based portal, including the classic PKG report, plus streamlined patient management tools. A companion smartphone app supports patients with medication reminders and logging, automatic data uploads, and simple troubleshooting.
The launch marks the expansion of Empatica’s platform into clinical care, building on its strong foundation in clinical trials, academic research, and digital biomarker development. For clinical research teams, the combined offering provides a solution that can seamlessly transition patients across clinical trials and clinical care, including integrated medication reminders, functional tests, and a unified patient experience.
To learn more visit empatica.com/clinical-care/parkinsons or contact pkg@empatica.com.
About Empatica
Empatica Inc. is a pioneer in continuous, unobtrusive remote health monitoring driven by AI. Empatica's FDA-cleared platform and technology are used by thousands of institutional partners for research purposes, in studies examining stress, sleep, epilepsy, migraine, depression, addiction, and other conditions. Its flagship medical wearable, EmbracePlus, has been developed with key partners including HHS, USAMRDC, and the NASA-funded TRISH. Its latest device, EmbraceMini, is the world’s smallest actigraphy device for use in clinical care and research. Empatica is also a global leader in patient-centric wearable epilepsy monitoring. In 2025, Empatica acquired PKG Health, expanding its offering into Parkinson’s Disease.
Scientific references:
- Nahab FB, Abu-Hussain H, Moreno L. Evaluation of Clinical Utility of the Personal KinetiGraph in the Management of Parkinson’s Disease. Advances in Parkinson’s Disease. 2019;8:42–61.
- Joshi R, Bronstein JM, Alcazar J, et al. An Observational Study of PKG Movement Recording System Use in Routine Clinical Care of Patients with Parkinson’s Disease. Frontiers in Neurology. 2019.