FDA Clears Empatica’s EmbraceMini for Sleep Monitoring
Boston, MA — Empatica, a pioneer in digital biomarker development and patient monitoring using wearables and AI, today announced that EmbraceMini has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
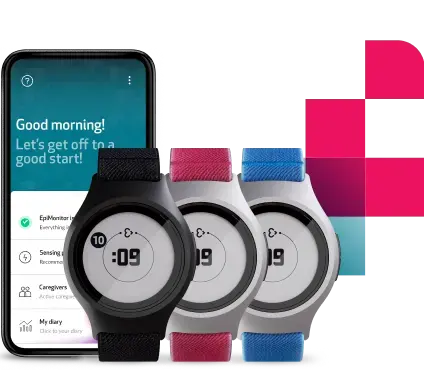
EmbraceMini was created to address the growing demand for patient-friendly, trustworthy, and scalable actigraphy solutions in clinical trials, especially where reliable and comfortable sleep monitoring is involved. The FDA clearance establishes EmbraceMini as a medical-grade wearable for remote health monitoring, enabling healthcare professionals and researchers to derive high-quality sleep and circadian rhythm assessments from real-world settings. In addition to sleep-related monitoring, the device supports broader analysis of physical activity, including use in clinical research and care contexts where quantifiable assessment of movement is important.
“The 510(k) reinforces Empatica’s commitment to developing scientifically rigorous and regulatory-aligned wearable technologies for monitoring health,” said Simone Tognetti, CTO and Co-founder at Empatica. “By clearing EmbraceMini alongside our already FDA-cleared Empatica Health Monitoring Platform, we strengthen the foundation for robust, continuous sleep and activity insights from ambulatory patients and trial participants, within an infrastructure built for secure data management and analysis. We have already seen the value EmbraceMini brings to trial sponsors through its compact form factor and ease of use, and we are excited to soon expand its role to clinical care, especially alongside our upcoming PKG offering for Parkinson’s.”
With this clearance, Empatica further grows its portfolio of FDA-cleared tools supporting clinical research, drug development, and remote physiological monitoring. EmbraceMini’s compact form factor and suitability for extended home use strongly align with the needs of decentralized and hybrid trials as well as modern clinical care practices, where continuous monitoring of sleep and physical activity is increasingly used to better understand disease impact and treatment effects in real-world settings across therapeutic areas ranging from obesity and depression to neurological and sleep disorders.
For more information, visit empatica.com/embracemini or reach out to our team.
About Empatica
Empatica Inc. is a pioneer in continuous, unobtrusive remote health monitoring driven by AI. Empatica's FDA-cleared platform and technology are used by thousands of institutional partners for research purposes, in studies examining stress, sleep, epilepsy, migraine, depression, addiction, and other conditions. Its flagship medical wearable, EmbracePlus, has been developed with key partners including HHS, USAMRDC, and the NASA-funded TRISH. Its latest device, EmbraceMini, is the world’s smallest actigraphy device for use in clinical care and research. Empatica is also a global leader in patient-centric wearable epilepsy and Parkinson’s monitoring.