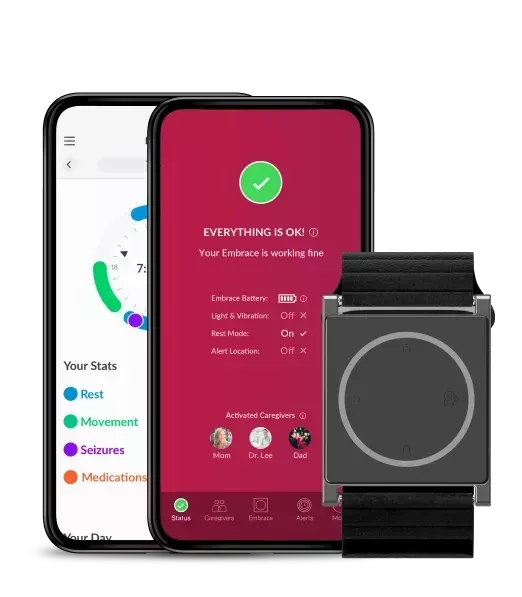
Empatica’s 2019 in review
It’s been an empowering year for our team, our trusted partners, and beloved customers.
As this 2019 comes to an end, we would like to look back with you all at the important milestones achieved together in the last 12 months.
May you enjoy the read (and the ride).
January: FDA clearance for children
The year could have not started any better. Exactly one year after receiving its first FDA clearance for seizure monitoring in adults, earlier this January Embrace2 received FDA clearance for children ages 6 and up, making it the first wrist-worn wearable in the field of epilepsy to be cleared as a medical device for children.
For families, this means that they can monitor their child’s tonic-clonic seizures directly at home and receive immediate notification when assistance is needed.
Physicians will be able to enhance the quality of monitoring by offering non-invasive solutions that easily integrate into their patient’s lives and offer them more freedom.
February: New partnership with the U.S. Government through DRIVe
Since the outset, we’ve been focused on developing breakthrough technology to improve and possibly save lives. The majority of our work has been on epilepsy, but others have seen the quality of Empatica devices and have approached us to help them in other medical areas as well.
Proudly, we’ve partnered with DRIVe, a division of The U.S. Department of Health and Human Services, to work on a research project aimed to develop a new smart watch to alert users when they’re developing a serious lung infection, even before any symptoms appear.
To do this, we have started investigating the use of the same advanced machine learning technology housed in our FDA-cleared Embrace2, to monitor physiological signals that suggest the person is getting ill and subsequently alert users and their caregivers.

March: Our Co-Founder and Chief Scientist conquers TED.com
Rosalind W. Picard is Co-Founder and Chief Scientist at Empatica, Founder and Director of the Affective Computing research group at the MIT Media Lab, and a recently elected member to the National Academy of Engineering, one of the highest professional distinctions in the field.
In her inspirational TED talk "Why Build AI?", she recounts how her research team at the MIT Media Lab began working on a wearable that measured electrodermal activity (EDA), to soon discover that such body signal could be accurately used for seizure detection. The idea of Embrace was born.
Her talk, selected to be featured on TED.com’s homepage, marks an important milestone in the efforts to raise epilepsy awareness and understanding of SUDEP (Sudden Unexpected Death in Epilepsy) and to educate a larger audience on the importance of building the kind of AI that we want to see built: “The AI that makes everybody’s life better.”
April: Launch of a new prescription upload system
As a design-driven company we strongly believe that when a customer has to face any process, it should be simple.
With a patient-centered approach, we have designed a new prescription upload system that makes it easy for our U.S. customers to sign up, upload the prescription, and get their Embrace2 delivered. Through an easily accessible, mobile-to-desktop interface, customers are now guided through step-by-step instructions to upload all the necessary information for their prescription to be safely validated. Thanks to the integration of the National Provider Identifier (NPI), patients don't need to manually include all the practitioner info, saving precious time, avoiding possible confusion and errors.
May: Purchasing Embrace2 gets easier with FSA and HSA cards
In a constant effort to expand access to Embrace2 to all patients that can benefit from its seizure monitoring capabilities, irrespective of their background, earlier this May we've started accepting FSA and HSA cards for payment.
Purchasing Embrace2 and a subscription plan with an FSA and HSA cards is no different than paying with any other card. With simplicity and accessibility in mind, we’re continuing to explore ways to remove barriers that could make it hard to receive Embrace2.
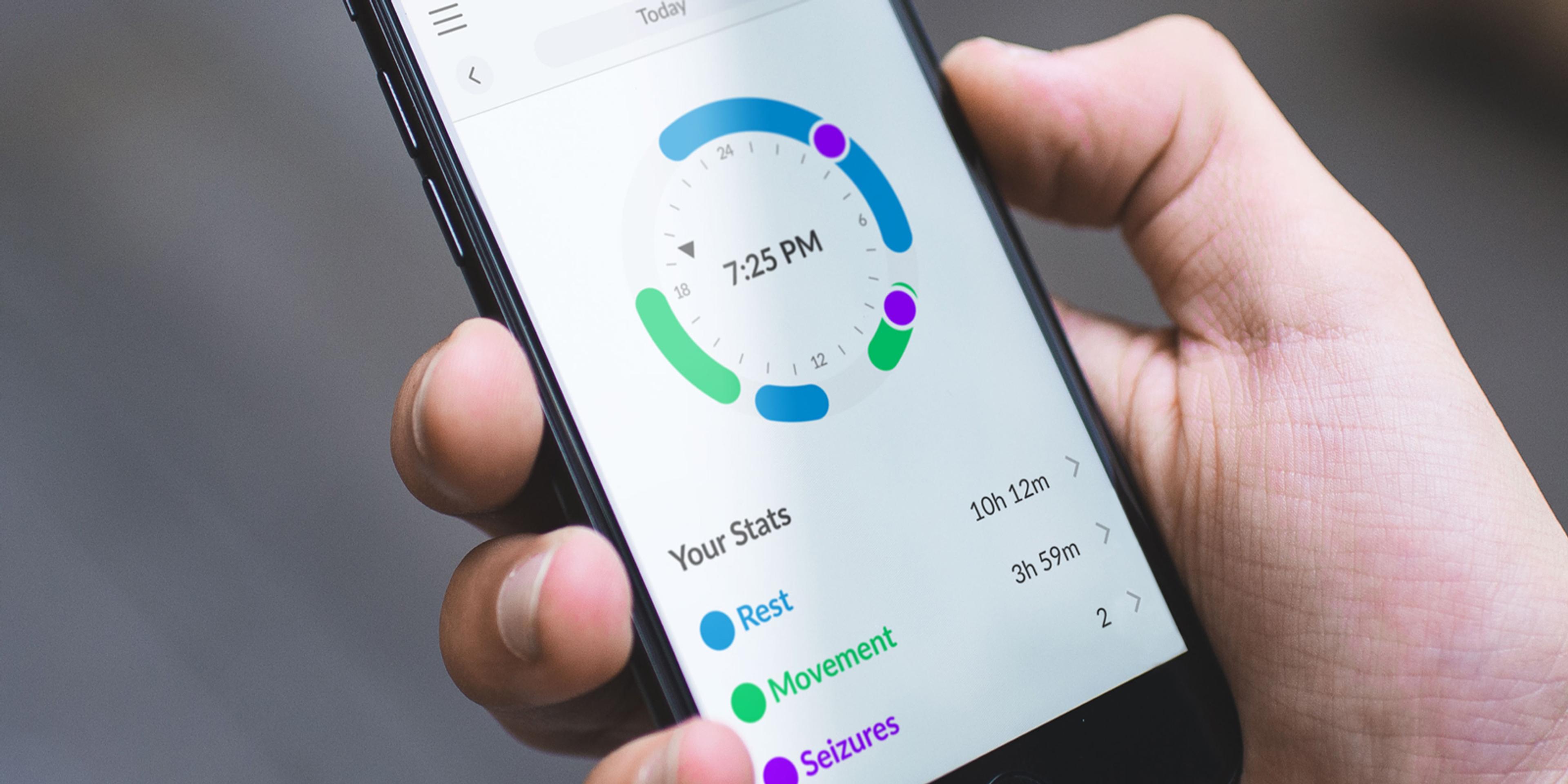
June: Release of a newly redesigned MATE app
Design is an ongoing process, a constant evolution to reflect our users’ changing needs.
Our latest version of the Mate App has been developed to offer a new design that is simple to understand, capable of crafting an engaging and meaningful experience while supporting our users in every step of their journey with epilepsy.
Mate, when used with Embrace2 and the Alert App, keeps track of the user’s rest, physical activity, and seizures. It’s an AI-powered seizure diary that automatically logs seizures detected by Embrace, with the possibility of manually adding relevant notes about each seizure. Having all of this information in one place allows users to easily notice any changes in their lifestyle and seizure frequency over time. And it also helps doctors identify possible triggers and understand how their patient is responding to treatment.
July: New partnerships with charity organizations
At the launch of the Embrace through our crowdfunding campaign on indiegogo, we donated about 1500 Embrace watches to the Epilepsy Foundation. Since then, we kept establishing partnerships with multiple charities. Most of these organizations have discount codes from Empatica, which can be given to people who want to purchase Embrace through them, while a few others can donate Embraces to individuals or families that need one, depending on the charity’s set requirements.
At Empatica, we understand that living with epilepsy can be a financial burden, and we’re committed to remove barriers and make significant advances in ensuring that Embrace gets in the hands of those that need it most.
August: Embrace2 becomes available in 12 more countries!
An estimated 65 million people worldwide currently live with epilepsy, and broadening the frontiers of our distribution network is always among our top priorities.
Since August, Embrace2 has become available in an additional 12 countries: Colombia, Cyprus, Ecuador, Guatemala, Honduras, Indonesia, Kuwait, Macao, Malta, Mozambique, Peru, Ukraine.
With these latest additions, our product’s reach is almost worldwide: from the Americas to Europe, Asia to Africa, Embrace is now used in 126 countries, supporting more and more people around the world.
September: First-time presentation of new algorithms
On September 6th, we attended the Second International Congress on Mobile Health Devices and Seizure Detection in Epilepsy, held in Lausanne, where our Data Scientists presented a validation study and the results of two new research projects.
Our FDA-cleared algorithm detected 12 out of 14 tonic-clonic seizures with a false alarm rate as low as 0.8 false alarms per 24 hours in a recent clinical prospective study. An offline version of our algorithm for the detection of generalized tonic-clonic seizures showed a reduction of false alarms up to 60%. The first algorithm developed for the detection of myoclonic seizures recognized 74% of the myoclonic seizures during the night, with a false alarm rate as low as 1 per night. Even though the results above are very promising, more needs to be done in order to see them out of the lab.
October: New partnership with NEC Corporation
We have partnered with NEC Corporation, the Japanese multinational information and network technology company, to use our Embrace2 in a revolutionary initiative to allow employees to monitor their stress levels using AI and smart wearables.
NEC uses the device's sensors in a study to estimate chronic stress by analyzing the physiological signals of voluntary participants.
Workplace stress has chronically been a large issue in Japan's culture. The goal of the initiative is to create a safe work environment, where employees can physically and mentally thrive, both within and outside the workplace. We are committed to supporting this important endeavor with our expertise in medical devices, software, and AI for healthcare.
November: Announcement of the EmbracePlus!
One of the major highlights of the year has been the announcement of our latest medical-grade wearable EmbracePlus, chosen by the Translational Research Institute for Space Health (TRISH) to be the first wearable to monitor the health of the astronaut crew that will be on board the first manned mission to Mars.
The device has been designed and developed to suit even the most advanced type of research needs, combining the best of Empatica’s existing research solutions and our consumer-focused, FDA-cleared monitor, the Embrace2. EmbracePlus will be available in 2020 to enable never-before-seen research breakthroughs in neurology and beyond.

December: Launch of Remote Seizure Monitoring
We are committed to bringing the latest, smartest, AI technology to help healthcare teams better understand their patients when deciding on treatments.
At the recent American Epilepsy Society’s (AES) Annual Meeting, in Baltimore, we announced a new service for healthcare providers called Remote Seizure Monitoring.
The new solution converts the data from our FDA-cleared, seizure detection algorithm of Embrace2 into a monthly report. The report is complete with detailed seizure, rest, and physical activity information to help doctors offer customized, data-driven care without having to rely on incomplete, hastily-put-together seizure diaries. And better yet, it is reimbursable by Medicare.
Our team has worked very hard in 2019 to provide patients, caregivers, clinicians, and researchers with the best in medical technology. We look forward to continuing to partner with you in 2020, as we innovate to improve health outcomes and help bring peace of mind to thousands of people.