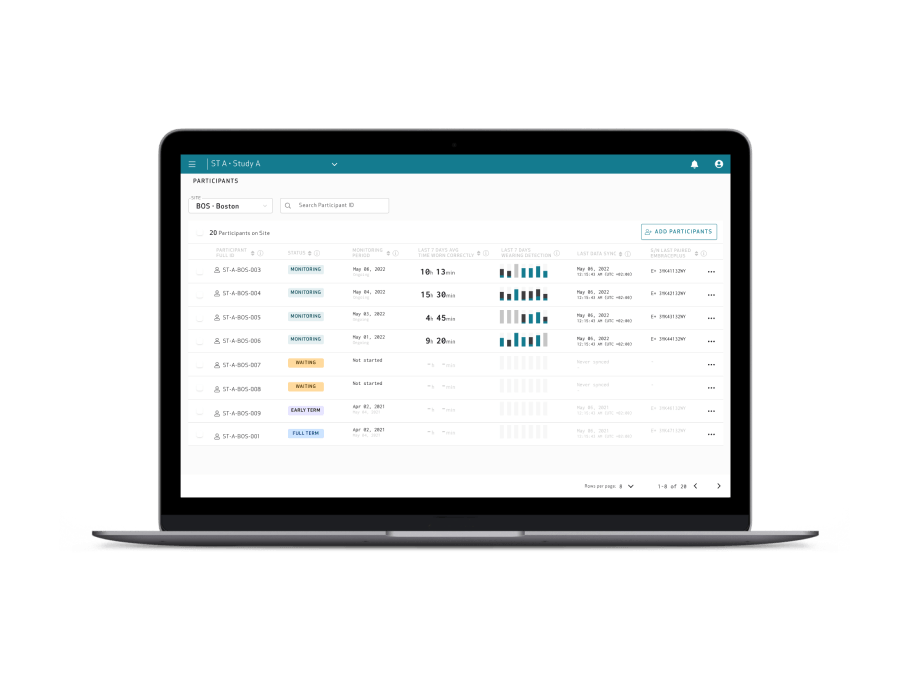
Empatica Health Monitoring Platform for Clinical Trials
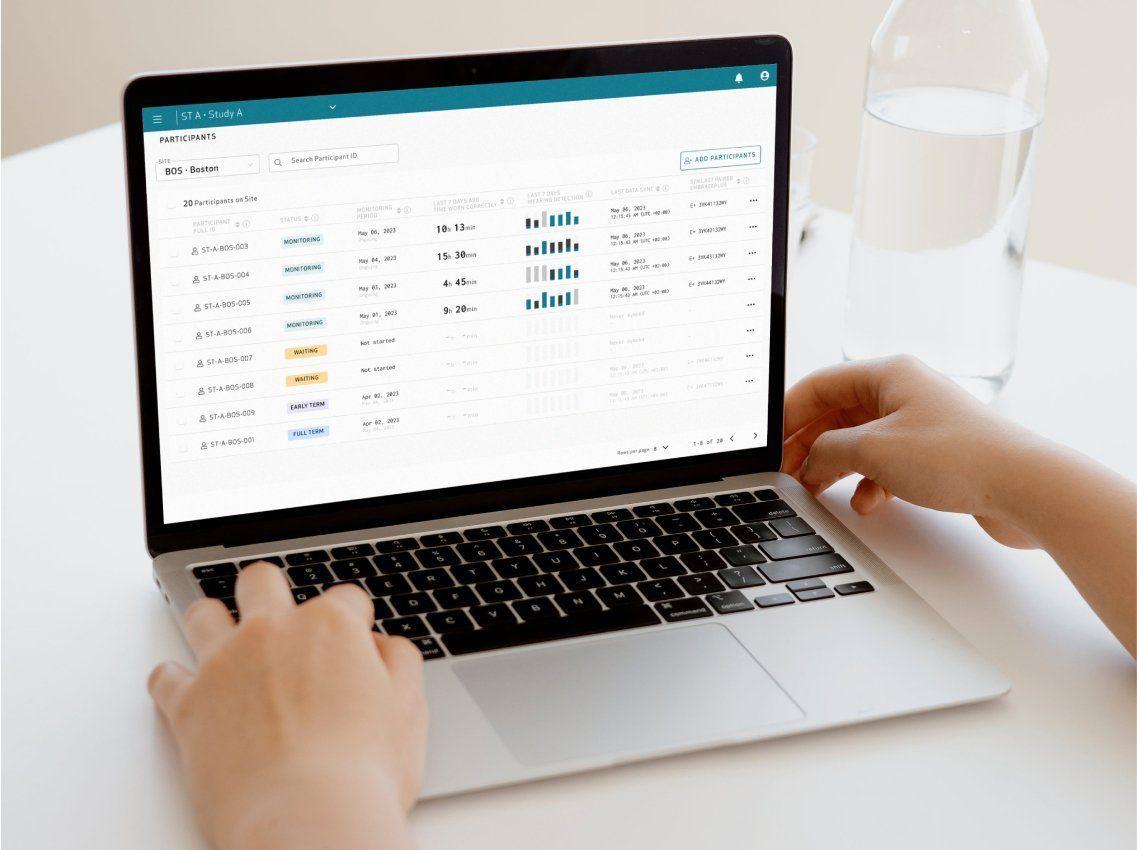

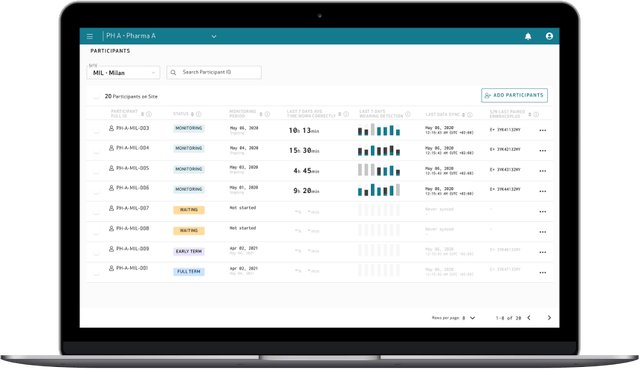
Enhance your clinical trials with raw data and digital biomarkers of the highest quality, with an easy-to-use, full-stack platform designed to reduce patient and site burden. Conducting research at scale from patients’ homes has never been easier.



Scalable

Modular

Reliable
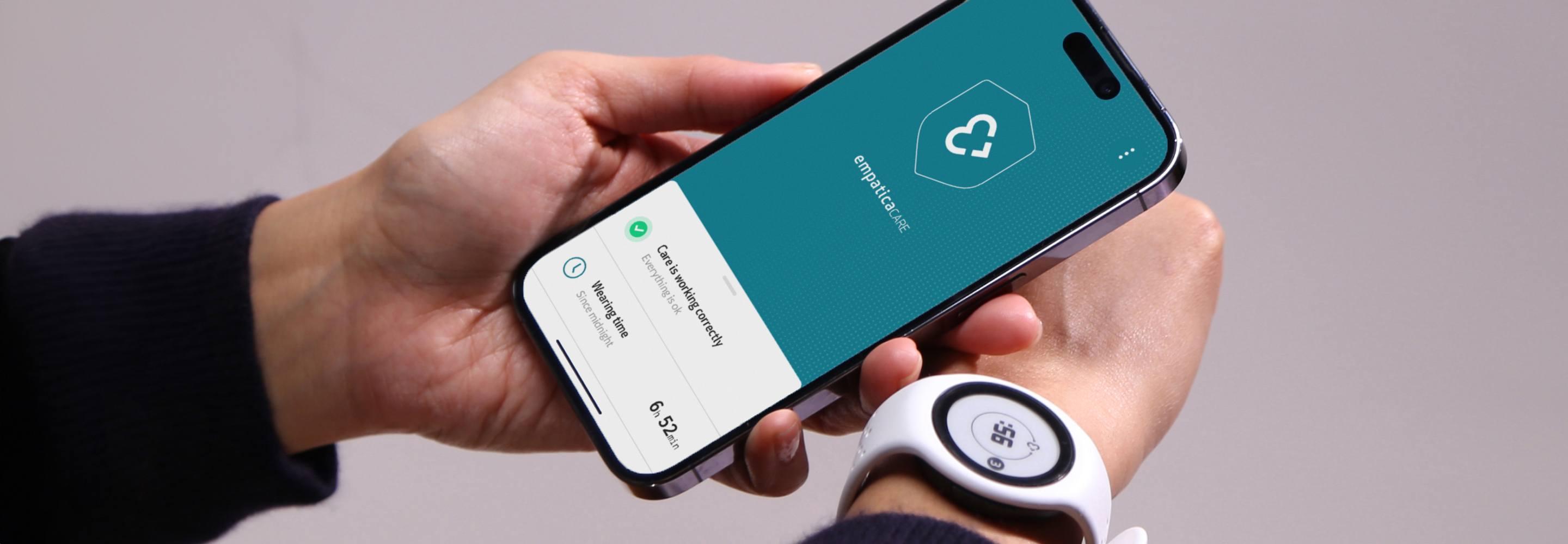

<2 months
from contract signing to starting data collection
>90% compliance
with EmbracePlus used as an actigraphy sensor
120+ Industry sponsored trials to date
have already implemented our Platform to collect data


Streamline clinical trials with Empatica’s Cloud API
Seamlessly integrate the Empatica Health Monitoring Platform into your existing clinical trial management system (CTMS) with our Cloud APIs.
- Reduce study complexities with seamless data flow
- Leverage Empatica’s 300+ digital measures and FDA-cleared medical wearable
- Manage EmbracePlus users directly from your own software
Learn more
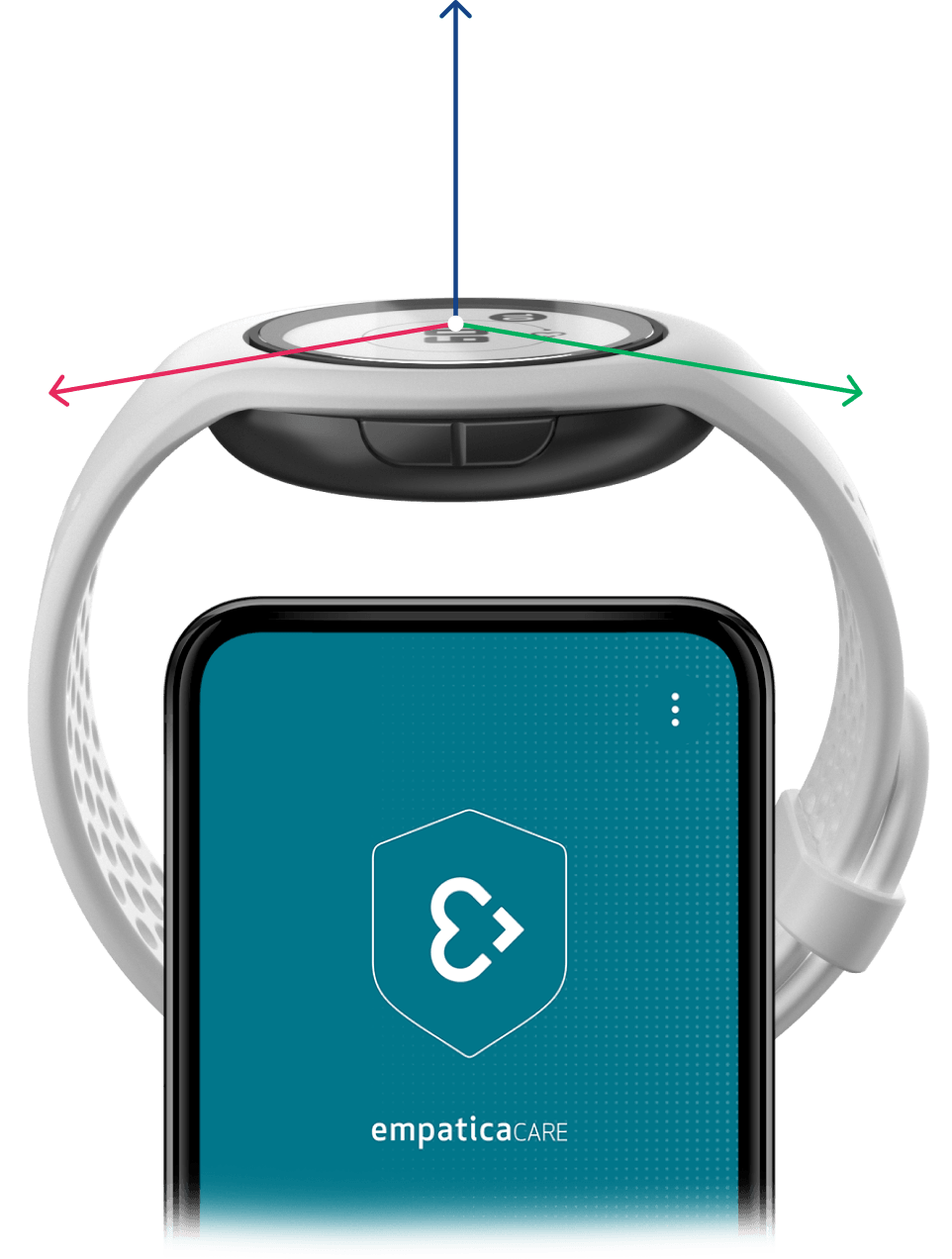

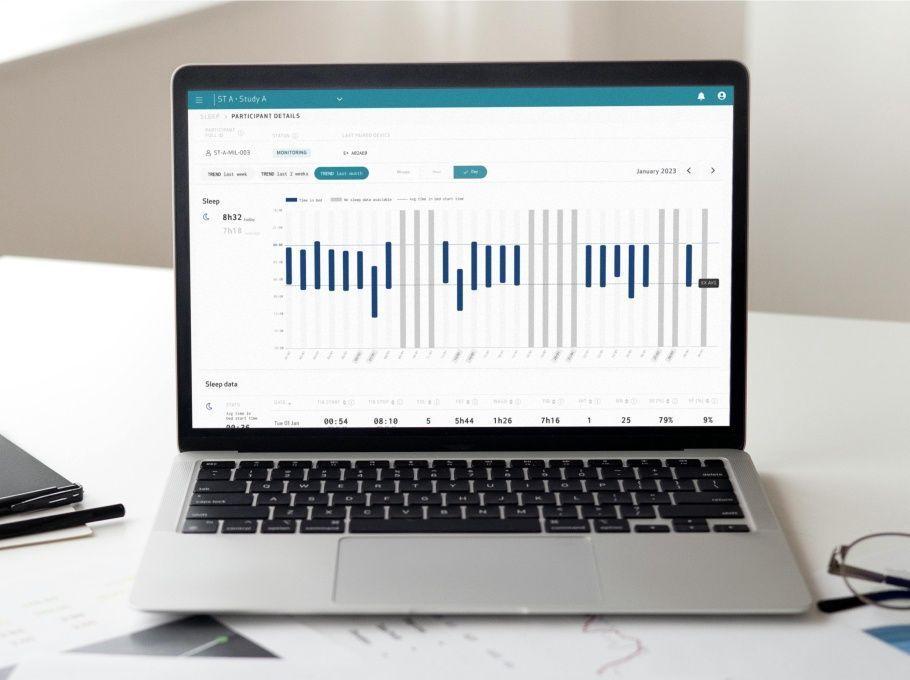
Empatica Actigraphy
Explore our full suite of activity-based digital biomarkers, available with our Actigraphy sensor modes
Learn more


Regulatory and Compliance
Empatica’s constant and decisive efforts aim at ensuring the safety, effectiveness, and quality of its medical devices as well as providing products that meet customer and applicable statutory and regulatory requirements
View Regulatory & Compliance