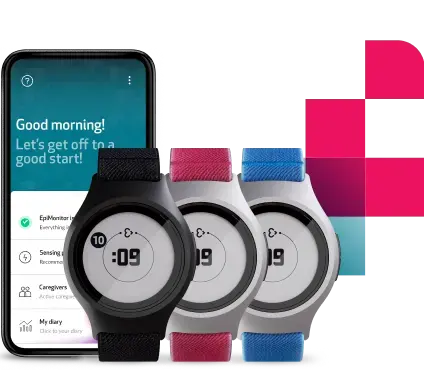
Achieve richer, more integrated data with eCOA
Today’s clinical trials require a unified patient view that combines physiological, behavioral, and subjective insights. The Empatica Health Monitoring Platform now integrates an advanced Electronic Clinical Outcome Assessment (eCOA) offering, enabling study teams to seamlessly capture performance outcomes and patient-reported outcomes to complement physiological data collected from Empatica’s wearables.
By embedding eCOA within a trusted ecosystem for passive monitoring, we have ensured a frictionless experience for both patients and clinical sites, while providing a richer dataset that can enhance the potential of modern clinical trials.
Pair ePROs, Functional Tests and Medication Reminders with the world’s largest set of digital measures in clinical research to gain a holistic patient view, from subjective experience to objective physiology, through a single, FDA-cleared system.
What eCOAs are included in the Empatica Health Monitoring Platform?
Functional Tests: Objective, repeatable assessments, performed with Empatica wearables
Functional tests transform traditional, in-clinic performance measures into digital, sensor-based assessments, conducted through the Care App using Empatica’s EmbracePlus and EmbraceMini devices. They provide quantitative, continuous, and repeatable measures of function, mobility, and tremor, delivered remotely, without additional burden for sites or participants.
ePRO: Patient insights, captured directly and digitally
ePROs can be easily configured for each study and deployed in multiple languages. All patient-reported data is stored alongside the physiological biomarkers collected through Empatica wearables, and is seamlessly accessible via the Care Portal for analysis and integration. Built for complementarity, ePRO enriches physiological signals with contextual patient input, enabling multidimensional insights. Our ePRO design springs from our expertise in building patient diaries that are being used in daily life by thousands of people, using our FDA-cleared epilepsy monitoring solution, EpiMonitor.
Medication reminders: Streamlining adherence for patients and sites
Automated medication reminders via the Care app address both the need of patients to be reminded to take their medication, and the need of clinicians to verify their adherence to treatment plans and trial protocols. Leveraging the platform-based approach developed by Empatica, the medication reminder feature can be customized based on specific needs, adapting to the different contexts, whether it’s for a clinical trial or for clinical care.
Designed for compliance, comfort, and clarity
Empatica’s modern and flexible Platform architecture allows rapid deployment while minimizing patient burden by bringing all interactions into a single, intuitive Care App experience.
All eCOA modules are directly integrated into the Care App, providing a single tool for patients for trial participation, data sharing, and system troubleshooting.
- Low burden: Patients interact with just one app and device
- Intuitive design: Familiar UX reduces training and improves adherence for both patients and sites
- Guided workflows: Clear, in-app step-by-step instructions for every test and questionnaire
- Real-time feedback: Automatic progress tracking and compliance reminders
Value across therapeutic areas
eCOA has benefits in multiple therapeutic areas, by pairing subjective patient experience with objective physiological data.
Oncology
eCOAs can be used to assess quality of life, pain, and fatigue tracking, which are all essential for cancer treatment assessment. The FDA specifically issued guidance recommending the use of PROs in oncology trials, as they may be important indicators of a patient’s quality of life.
CNS & Neurodegenerative Disorders
In conditions such as Depression, Alzheimer's, and Parkinson's, both subjective and objective measures are important. Digital cognitive assessments combined with patient diaries track disease progression and treatment response with greater sensitivity.
Cardiology
Digital biomarkers from wearables correlate with patient-reported dyspnea and activity limitations, creating powerful endpoints for heart failure trials.
Respiratory (COPD, Asthma)
Environmental triggers, medication usage, and symptom burden tracked daily provide actionable insights for managing chronic respiratory conditions.
Rheumatology (Arthritis, Lupus, other autoimmune conditions)
Pain levels, mobility assessments, and patient-reported flares help quantify disease activity between clinical visits.
Endocrinology
Diabetes management combines glucose monitoring with lifestyle and symptom reporting, eg dietary intake. eCOA supports more comprehensive metabolic profiling, helping aligning treatment plans with real-world patient behavior.
Gastroenterology (IBD, IBS)
eCOA enables high-frequency, low-friction logging of patient-centered symptoms that can vary significantly day-to-day, such as abdominal pain reporting, stool-consistency logs, and flare tracking. These are all central to evaluating treatment performance.
Dermatology
Itch intensity, pain, sleep disruption, and self-reported skin discomfort often correlate strongly with disease impact.
Integrated data can lead to more efficient studies and improved compliance
eCOAs can be easily configured for each study and deployed in multiple languages. All patient-reported data is stored alongside the physiological biomarkers collected through Empatica wearables, and is seamlessly accessible via the Care Portal for analysis and integration.
Gain access to richer data sets, faster insights, and simplified operational workflow, all from one trusted solution:
- Unified data pipeline: ePRO, ePerfO, and digital biomarkers captured in one ecosystem
- Regulatory-grade compliance: HIPAA/GDPR compliant, part of FDA-cleared and CE MDR certified platform
- Accelerated timelines: Streamlined build and deployment for global studies
- Global readiness: Pre-validated instruments and multilingual support
By unifying eCOA with continuous wearable sensor data inside one secure platform, Empatica empowers clinical researchers to generate richer, more actionable insights, without any added complexity for patients and sites. Talk to our team to see how you can introduce a fully integrated approach in your trials today.