Wondering if our solutions and digital biomarkers fit your needs?
Reach out and a member of our team will be in touch with you as soon as possible.
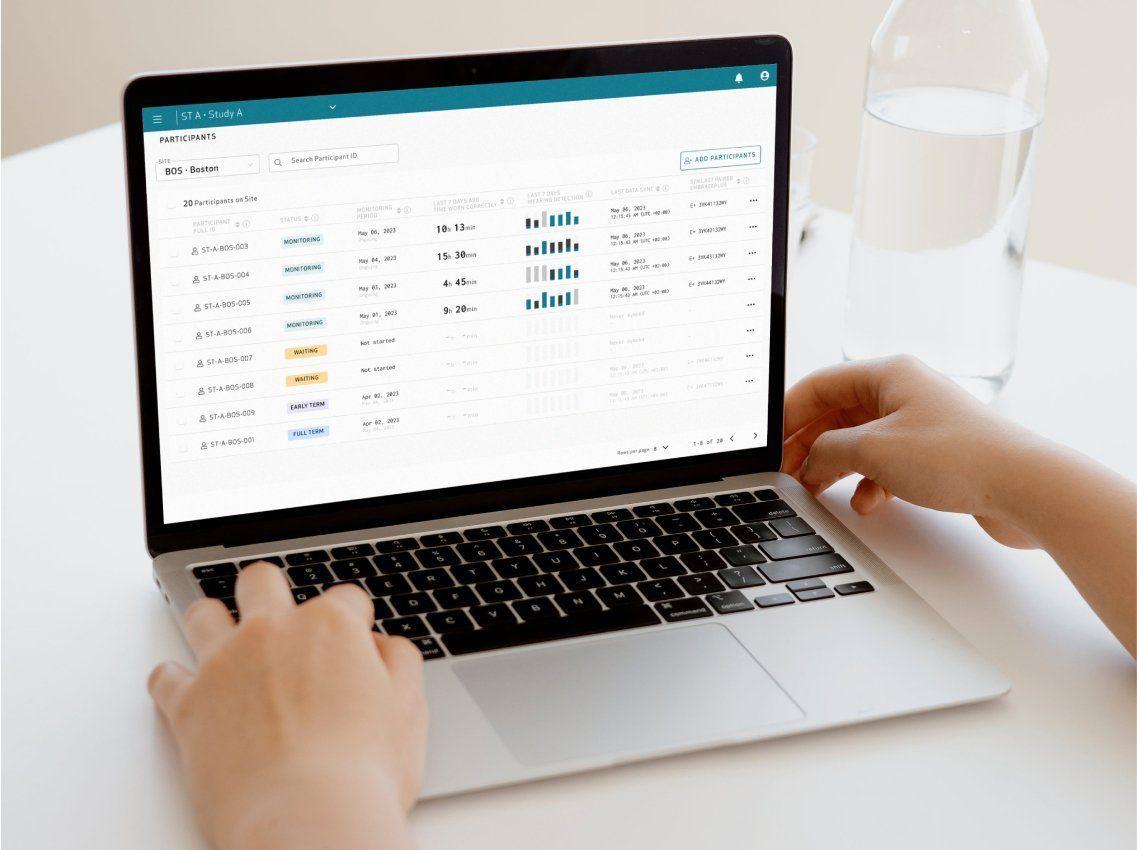







from contract signing to starting data collection
with EmbracePlus used as an actigraphy sensor
have already implemented our Platform to collect data


Seamlessly integrate the Empatica Health Monitoring Platform into your existing clinical trial management system (CTMS) with our Cloud APIs.

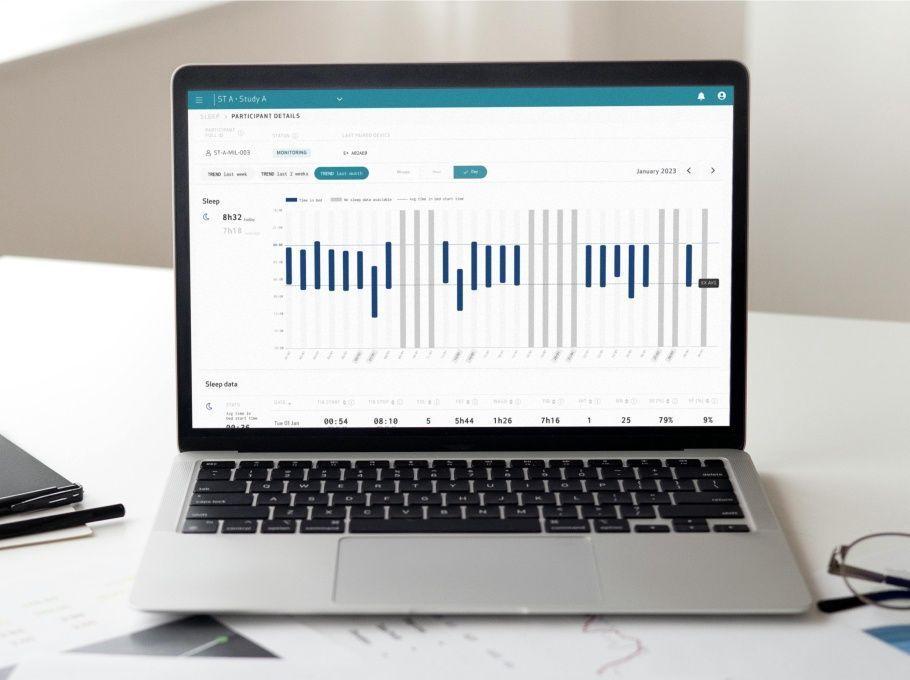
Explore our full suite of activity-based digital biomarkers, available with our Actigraphy sensor modes


Empatica’s constant and decisive efforts aim at ensuring the safety, effectiveness, and quality of its medical devices as well as providing products that meet customer and applicable statutory and regulatory requirements










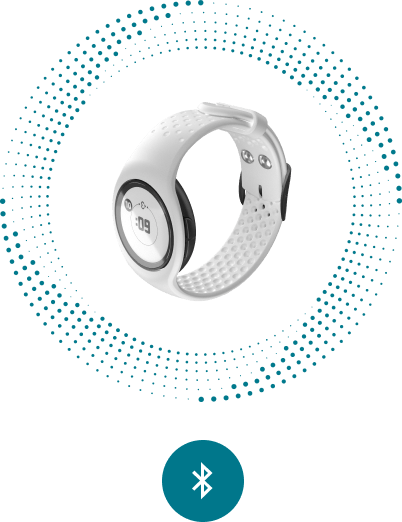
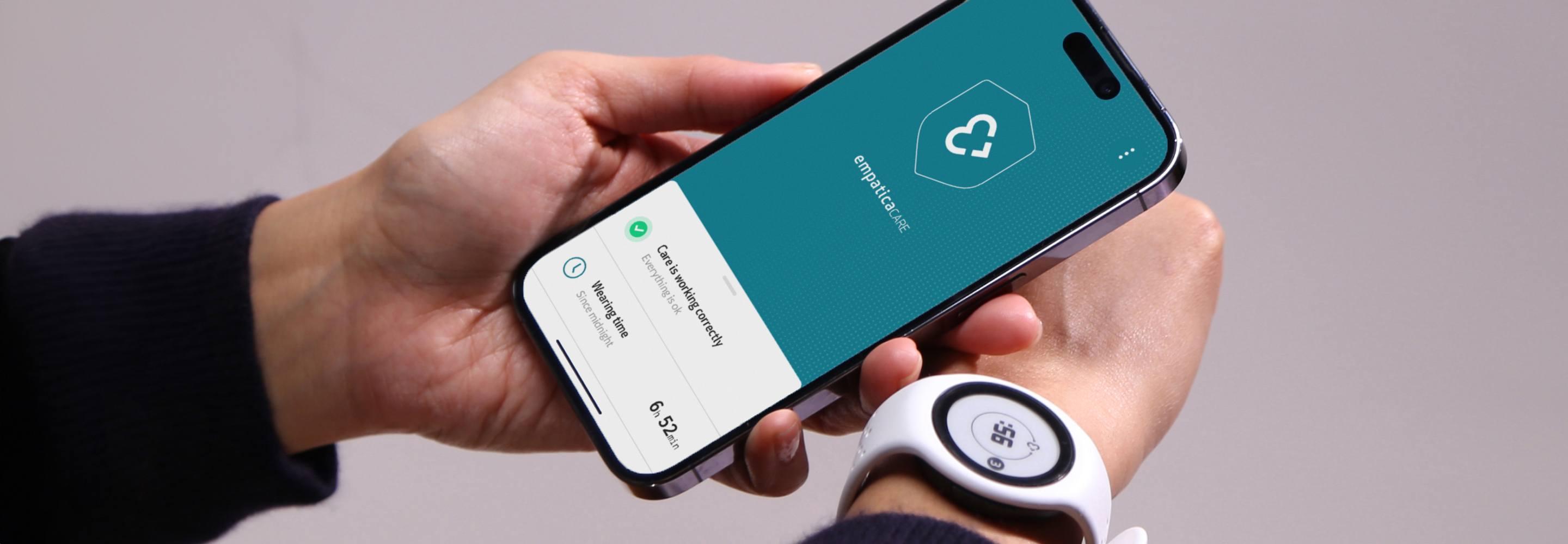
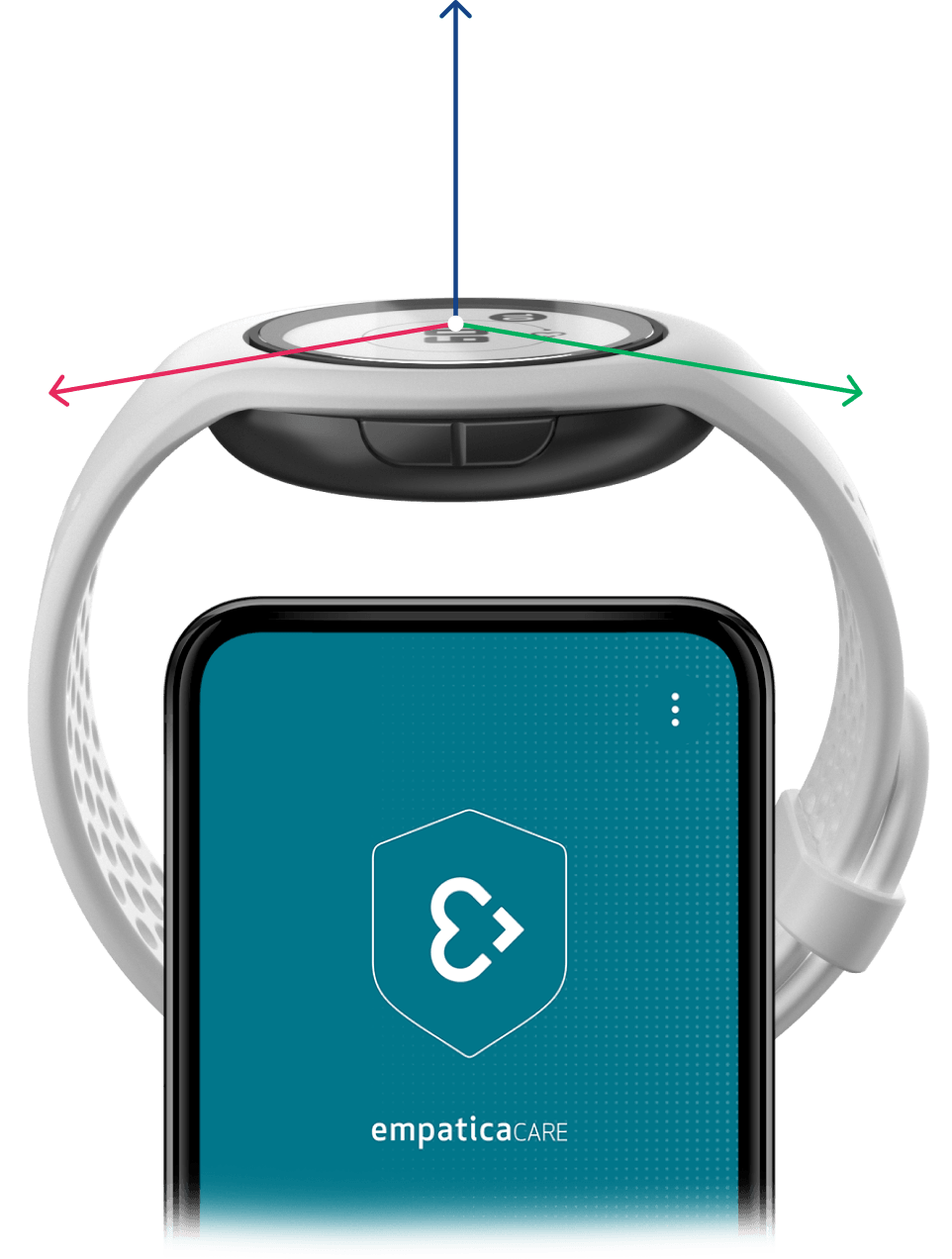
EmbracePlus
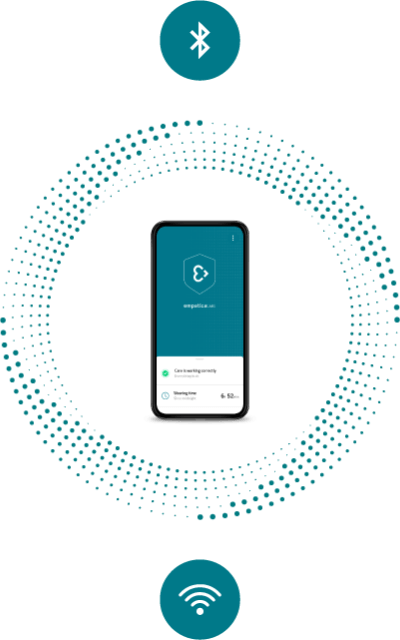
Care App

Empatica Cloud
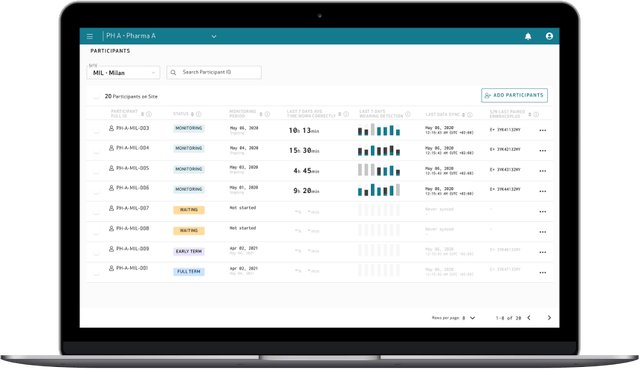
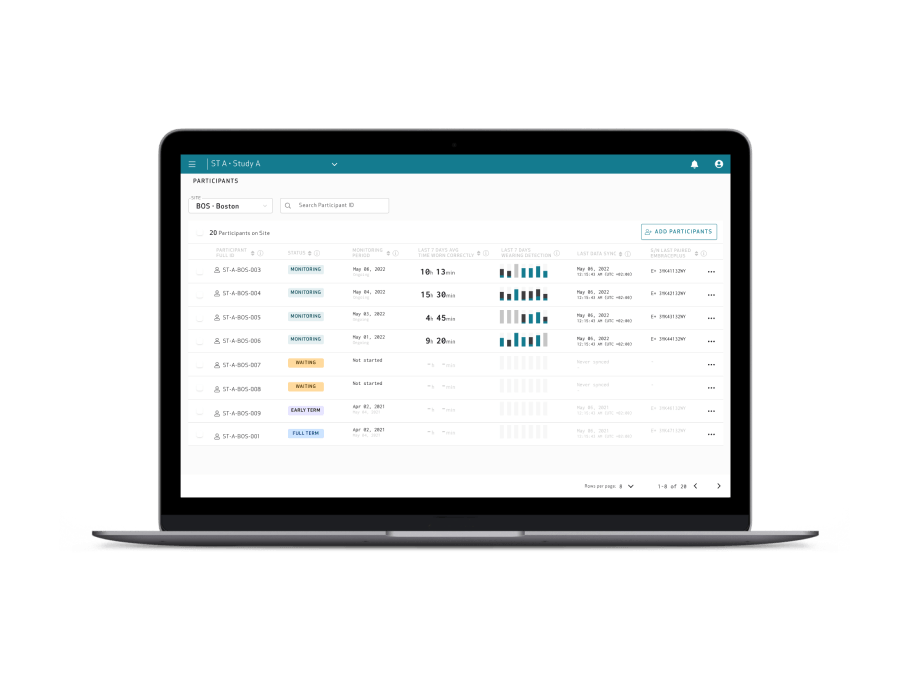
Care Portal

EmbracePlus

Care App

Empatica Cloud
Your Cloud